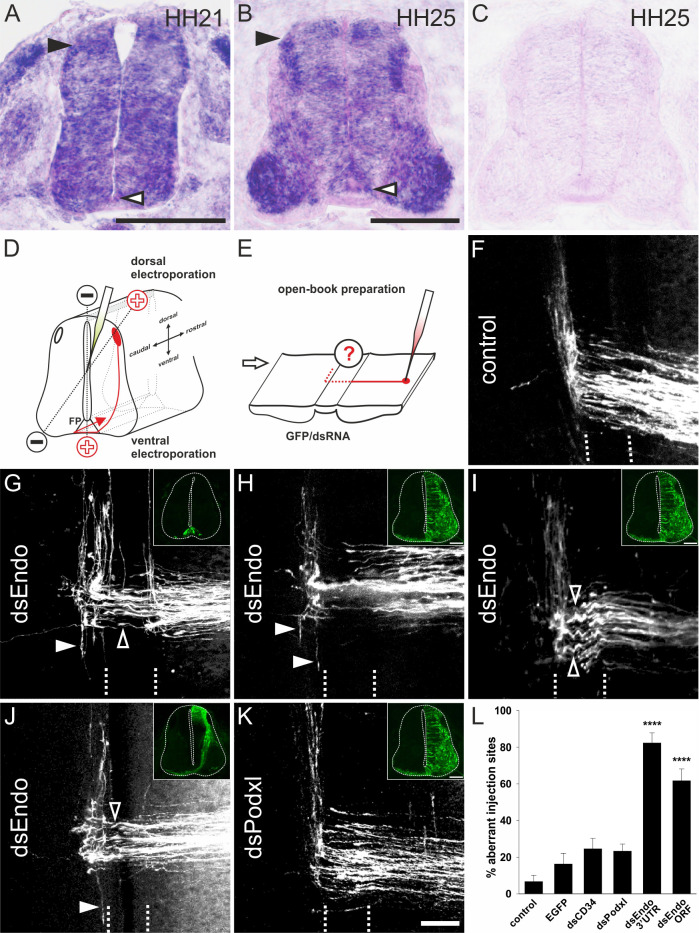
Figure 2. Endoglycan is required for correct turning of post-crossing commissural axons.
(A,B) Endoglycan is expressed in the developing neural tube during commissural axon guidance. Endoglycan is expressed throughout the neural tube at HH21, including the floor plate (white arrowhead) (A). (B) At HH25, Endoglycan is still found in most cells of the spinal cord. High levels are found in motoneurons and interneurons, including the dorsal dI1 neurons (black arrowhead), and in the floor plate (white arrowhead). No staining was found when hybridization was carried out with a sense probe (C). Commissural axon pathfinding was analyzed in ‘open-book’ preparations (D,E; see Materials and methods for details). The positions of the electrodes for dorsal and ventral electroporation are indicated (D). In control embryos at HH26, commissural axons have crossed the floor plate and turned rostrally along the contralateral floor-plate border (F). In contrast, after downregulation of Endoglycan (G–J) commissural axons failed to turn along the contralateral floor-plate border or they turned randomly either rostrally or caudally (arrowheads in G-J). Occasionally, axons were turning already inside the floor plate (open arrowhead in G). A closer look at the morphology of the axons in the floor plate revealed their tortuous, ‘corkscrew'-like trajectory across the midline at many DiI injection sites (open arrowheads in I). To knockdown Endoglycan either in the floor plate or in commissural neurons, the ventral or dorsal spinal cord was targeted as indicated in (D) (see inserts in G and J, respectively). Phenotypes were the same as those observed after targeting one half of the spinal cord including the floor plate (H,I). Pathfinding was normal in embryos electroporated with dsRNA derived from Podocalyxin (K). The quantification of injection sites with pathfinding errors after targeting the floor plate or one half of the spinal cord is shown in (L). Pathfinding errors were seen only at 6.7±3.4% of the injection sites in untreated control embryos (n=10 embryos, 45 injection sites). In control embryos injected and electroporated with the EGFP plasmid alone, pathfinding errors were found at 16.2±6% of the injection sites (n=17 embryos, 92 injection sites). Injection and electroporation of dsRNA derived from CD34 (24.6±5.8%, n=8 embryos, 80 injection sites) and Podocalyxin (23.3±3.9%, n=17 embryos, 147 injection sites) did not affect midline crossing and turning behavior of commissural axons. By contrast, 82.3±5.6% (n=11 embryos, 65 sites) and 61.7±6.4% (n=18, 161 sites) of the injection sites in embryos injected with dsRNA derived from the 3’-UTR or the ORF of Endoglycan, respectively, showed aberrant pathfinding of commissural axons. One-way ANOVA with Tukey’s multiple comparisons test. P values ****<0.0001, compared to EGFP-injected control groups. The two groups electroporated with dsRNA derived from Endoglycan were not different from each other. Values represent average percentage of DiI injection sites per embryo with aberrant axonal navigation ± standard error of the mean. Source data and statistics are available in the Figure 2—source data 1 spreadsheet. Bar: 50 μm.
Figure 2—figure supplement 1. Endoglycan is mainly expressed in the developing nervous system.
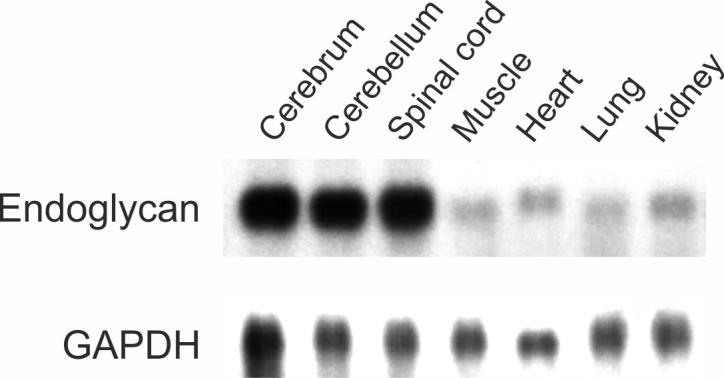
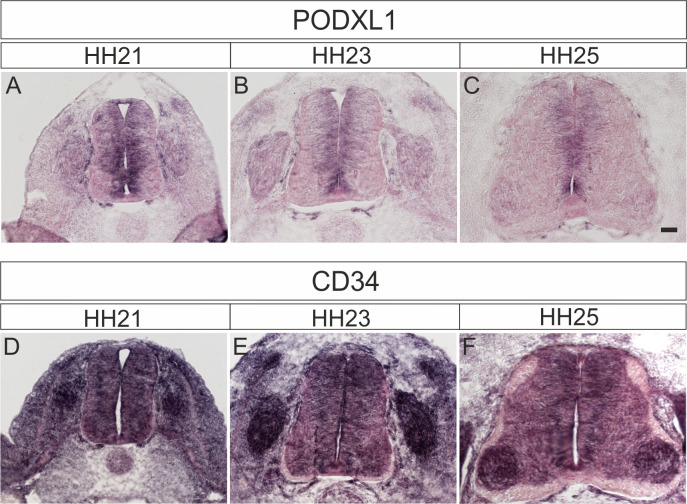
Figure 2—figure supplement 2. Podocalyxin and CD34 are expressed in the developing spinal cord during midline crossing of dI1 commissural axons.
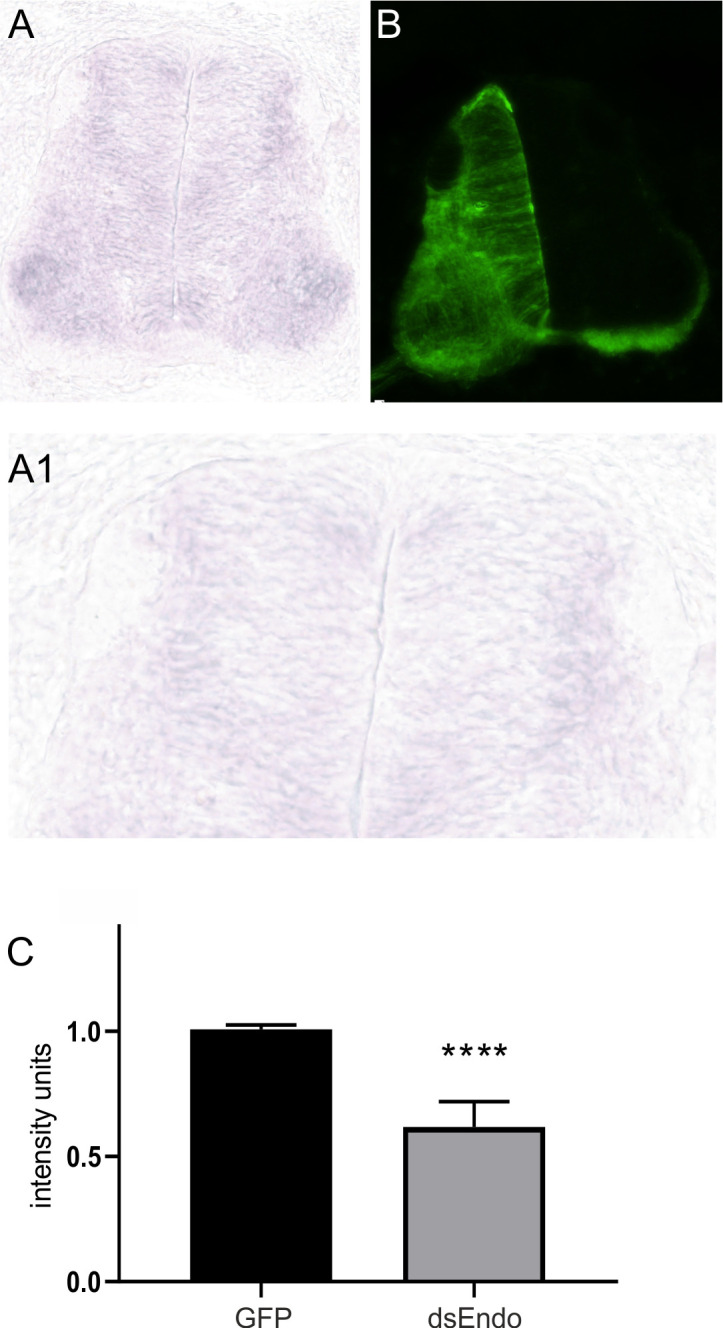
Figure 2—figure supplement 3. Electroporation of dsRNA derived from Endoglycan effectively downregulates Endoglycan mRNA.