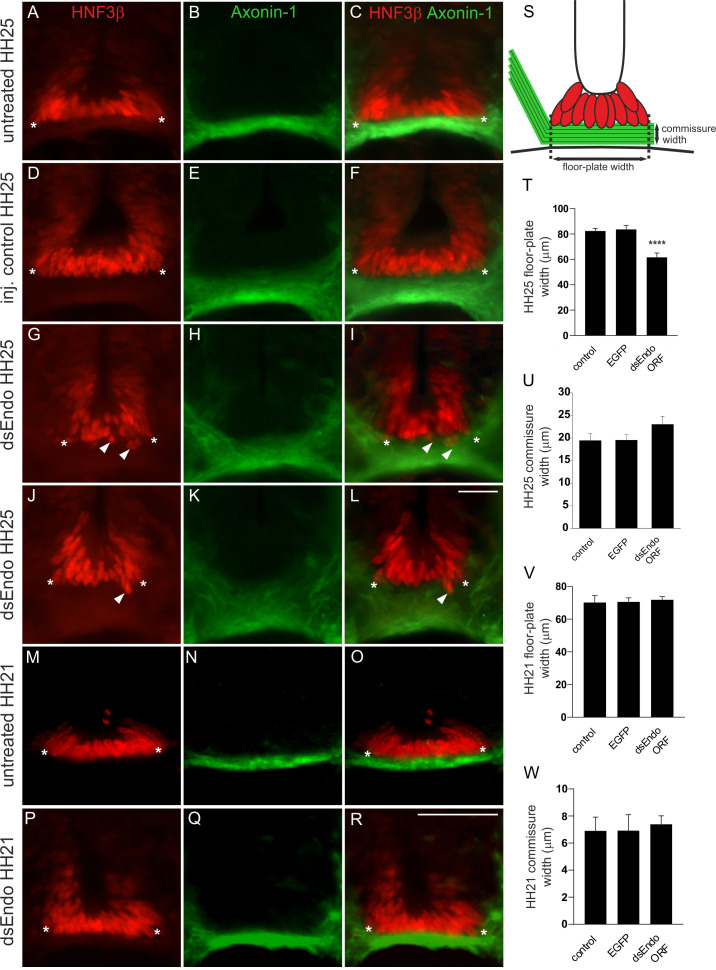
Figure 3. After downregulation of Endoglycan in one half of the spinal cord, the floor-plate morphology is compromised only after axonal midline crossing.
In untreated (A-C) and control-treated embryos (D-F), the floor plate is of triangular shape with floor-plate cells precisely aligned at the ventral border. There is no overlap between the floor plate (visualized by HNF3β staining; red) and the commissure (visualized by anti-Axonin1 staining; green). The shape of the floor plate is no longer triangular in embryos lacking Endoglycan (G-L). The floor-plate cells are not aligned ventrally (arrowheads in G, I, J, and L) and the floor plate appears to have gaps. This change in morphology is only seen at HH25, when midline crossing is completed. When the floor-plate morphology was analyzed at HH21, there was no difference between control (M-O) and experimental embryos electroporated with dsRNA derived from Endoglycan (P-R). Note that some more ventral commissural axon populations have crossed the floor plate at this stage. But overall, the number of axons that form the commissure at HH21 is still very small. The width of the floor plate (indicated by asterisks) was measured (S,T). There was no significant difference in spinal cord width at HH25 (400.2 ± 54.5 µm in untreated controls, 438.2 ± 30.3 µm in EGFP-expressing controls, and 394 ± 12.0 µm in dsEndo embryos), but floor plates were significantly narrower in embryos lacking Endoglycan (T; 61.6 ± 2.9 µm; n = 7 embryos; p<0.001) compared to untreated (82.4 ± 1.8 µm; n = 6 embryos) and EGFP-injected control embryos (83.6 ± 2.6 µm; n = 6 embryos). One-way ANOVA with Tukey’s multiple comparisons test. The commissure had a tendency to be wider in experimental compared to control embryos but the effect was not statistically significant (U). The width of the floor plate was not different between groups when measured at HH21 (V) with 70.1 ± 4.2 µm (n = 3) for untreated and 70.6 ± 2.5 µm for EGFP controls (n = 4), compared to 71.8 ± 2.0 µm for experimental embryos (n = 3). No difference was seen in the width of the commissure. Two-tailed T-test. ****p<0.0001. Mean ± SEM are given. Electroporation was targeted to one side of the spinal cord as shown in the insert in Figure 2H. Bar: 50 µm. Source data and statistics are available in Figure 3—source data 1 spreadsheet.
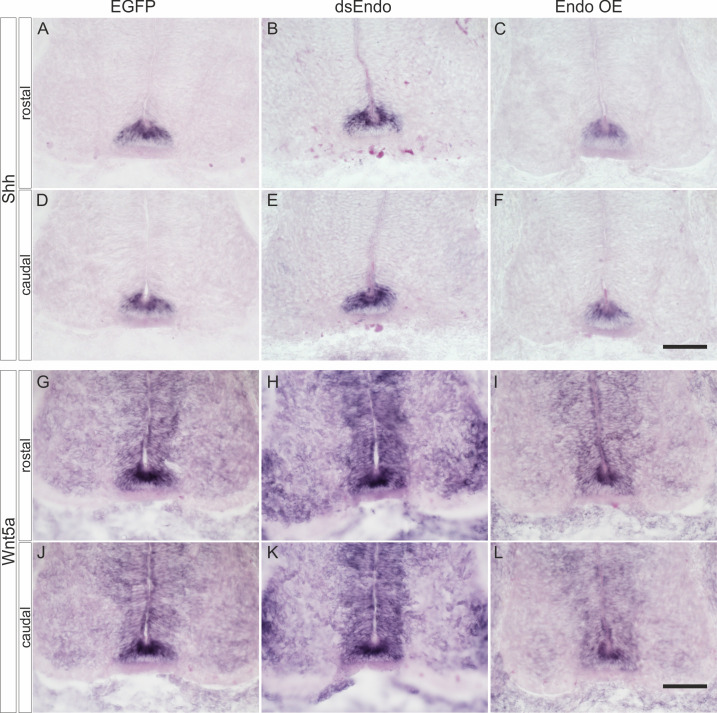
Figure 3—figure supplement 1. Downregulation or overexpression of Endoglycan does not affect spinal cord patterning.
Figure 3—figure supplement 2. Experimental manipulation of Endoglycan levels does not induce cell death in the floor plate.