Abstract
Background.
Exposure to endocrine disrupting chemicals (EDC), such as phthalates and phenols, during pregnancy may be associated with excessive gestational weight gain (GWG), an important predictor of future health of the mother and the offspring. There is however a paucity of literature examining this association, and no study has accounted for the complex nature of EDCs exposure as a time-varying mixture of chemicals.
Objective.
We examined the association between trimester-specific EDCs mixture and GWG in pregnant women attending a fertility clinic, to identify windows of susceptibility to such exposures, and assess the individual contribution of each chemical over pregnancy.
Methods.
We included 243 pregnant women from the Environment and Reproductive Health (EARTH) Study, who provided up to 3 urine samples (one per trimester), and with available data on GWG. Urinary concentrations of 7 phthalate metabolites, bisphenol A, and 2 parabens, corrected for specific gravity, were included in the analysis. The association between trimester-specific EDCs mixture and GWG was evaluated using multiple regression models – categorizing exposures into concentration quartiles– and with Bayesian Kernel Machine Regression (BKMR), while adjusting for potential confounders. Hierarchical BKMR (hBKMR) was used to account for the time-varying nature of chemical concentrations over pregnancy, identifying the most important trimester and most important EDC within each trimester.
Results.
During 1st trimester, higher GWG was observed at higher sum of metabolites of di(2-ethylhexyl) phthalate (ΣDEHP) from both multiple regression (e.g. comparing the 4th quartile with the 1st: β=2.36 kg, 95% CI:−0.47, 5.19) and BKMR. During 2nd and 3rd trimesters, positive associations with mono-n-butyl phthalate and propylparaben, and negative with ΣDEHP and methylparaben were observed. When evaluating exposures as a time-varying mixture with hBKMR, 1st trimester was the most important exposure window when evaluating prenatal urinary EDCs in relation to GWG. Within the 1st trimester, urinary ΣDEHP, mono-isobutyl phthalate and propylparaben had the highest contribution in the positive association between the mixture and GWG.
Conclusion.
We observed positive associations between urinary EDCs during pregnancy, especially DEHP metabolites, and GWG. Our results suggest the 1st trimester of pregnancy as the time window of highest susceptibility to the effects of EDCs on GWG, with potential indication for the design of public health interventions, informing prevention strategies for reducing sources of exposure at specific time points.
Keywords: Endocrine disruptors, gestational weight gain, environmental epidemiology, mixture modeling, pregnancy
1. INTRODUCTION
Excessive gestational weight gain (GWG) during pregnancy has been associated with adverse health outcomes in both mother and offspring, such as gestational diabetes, hypertensive disorders and preterm birth.1–3 In the United States, it is estimated that almost 50% of women gain more than the recommended amount of weight during pregnancy.4 High levels of leptin and progesterone may lead to the development of excessive GWG, thus suggesting that endocrine factors may play a role in the development of the condition.5,6 As such, exposure to endocrine disrupting chemicals (EDCs) during pregnancy, such as phthalates, parabens and bisphenol A (BPA), may lead to GWG outside of the recommended range.7,8 EDCs are ubiquitously found in common household items including personal care products, food and pharmaceuticals,9,10 yet the association between select EDCs and GWG in epidemiological studies has been studied only recently and with inconclusive results.11,12
Humans are exposed to several phthalates, phenols, and parabens, present in the real world as chemical mixtures.13 Moreover, these EDCs are non-persistent chemicals that are rapidly metabolized, and exposures are likely episodic over time.9 While the entire pregnancy is a critical period for both mother and fetus, it has been hypothesized that mothers may be particularly susceptible to chemical exposures during specific sensitive periods of pregnancy, where rapid weight and metabolic changes are occurring.14–16 The identification of specific windows of susceptibility for mothers during pregnancy, as well as the assessment of the individual contribution of different EDCs within mixtures of exposures, can assist in designing interventions and informing prevention strategies that would reduce sources of exposure at specific time points, such as early pregnancy rather than later.16 To identify such windows of susceptibility to EDCs, a study design with repeated assessment of environmental exposures is required, and statistical methods that allow evaluating such chemicals as time-varying exposures, possibly accounting for the complex nature of exposures as mixtures, are needed.17 Among other methods proposed to such end, extensions of the Bayesian Kernel Machine Regression (BKMR) framework allow incorporating the presence of time-varying mixtures of chemicals, as well as identifying the specific contribution of different chemicals at different time points.18
Therefore, in this study, we employed hierarchical BKMR (hBKMR) to examine the association between a trimester-varying mixture of EDCs and GWG in a cohort of pregnant women attending a fertility clinic, exploring windows of susceptibility to such exposures during pregnancy, and assessing the individual contribution of each chemical.
2. METHODS
2.1. Study population
We used data from the Environment and Reproductive Health (EARTH) Study, which is an ongoing, prospective cohort designed to identify environmental and dietary determinants of fertility and pregnancy outcomes among couples presenting to Massachusetts General Hospital (MGH) Fertility Center (Boston, MA). Women were eligible if they were age 18 to 45 years at enrollment. The details of the EARTH study have been described previously.19 The present analysis included women who contributed at least one urine sample during pregnancy and had their gestational weight recorded at both the first and the last obstetrics visit. Therefore, the present study consisted of 243 mothers. Out of the 243 women in this study, 240 had data on 1st trimester exposures, 209 on 2nd trimester, 178 on 3rd trimester, and 154 women had data available for each of the three trimesters. Trained research staff obtained informed consent and the study was approved by the Human Studies Institutional Review Boards of the Partners, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC).
2.2. Urinary sample collection and quantification of exposure biomarker concentrations
Biomarker concentrations were assessed in spot urine samples collected in sterile polypropylene cups during the women’s first (median: 7 gestation weeks), second (median: 21 gestation weeks), and/or third trimesters of pregnancy (median: 27 gestation weeks). Specific gravity was measured at room temperature using a handheld refractometer within several hours (typically within 1 h) after urine collection (National Instrument Company, Inc., Baltimore, MD, USA). The urine was divided into aliquots and frozen at −80 °C. Samples were shipped on dry ice overnight to the CDC (Atlanta, GA, USA).
Following standard procedures previously described,20,21 phthalates metabolites, parabens and BPA were quantified by on-line solid phase extraction coupled with high-performance liquid chromatography isotope dilution-tandem mass spectrometry. The limit of detection (LOD) was 0.5–1.2 μg/L for mono(2-ethylhexyl) phthalate (MEHP), 0.2–0.7 μg/L for mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), 0.2–0.6 μg/L for mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), 0.2–0.3 μg/L for monobenzyl phthalate (MBzP), 0.2–0.8 μg/L for mono-isobutyl phthalate (MiBP) and 0.4–0.6 μg/L for monon-butyl phthalate (MBP); 1.0 μg/L for methylparaben, and 0.1–0.2 μg/L for propylparaben; 0.1–0.4 μg/L for BPA. Because of their high levels of correlation, to reduce the risk of multicollinearity in our statistical models, di(2-ethylhexyl) phthalate (DEHP) metabolites were evaluated by taking their molar sum (ΣDEHP) and expressed in units of μmol/L. For this study we only included chemicals detected in more than 60% of samples: MiBP, MBP and MBzP, the four metabolites of DEHP: MEHP, MEHHP, MEOHP, MECPP; propylparaben, methylparaben; and BPA. All biomarker concentrations were corrected for SG using the formula Pc =P [(1.014−1)/(SG − 1)], where Pc is the SG-corrected concentration (μg/L), P is the measured concentration (μg/L), and 1.014 is the mean SG concentration in the study population included in the analysis. Concentrations below the LOD were assigned a value equal to the LOD divided by the square root of 2 prior to SG adjustment as described previously.22
2.3. Outcome assessment
We obtained gestational weight in kilograms at the first and the last obstetrics visit from hospital medical records. The outcome, gestational weight gain (GWG), was calculated as the difference between the recorded weight at the last and the first obstetrics visit. We evaluated GWG as a continuous outcome without any transformation.
2.4. Covariates assessment
Sociodemographic and lifestyle data, and family medical history were obtained from a brief questionnaire administered by the study staff at enrollment and a detailed take-home questionnaire. All statistical models were adjusted for the following a priori identified potential confounders based on the literature: maternal age (continuous, years), pre-pregnancy BMI (continuous, kg/m2), race (binary, white/non-white), self-reported smoking status (binary, ever/never), education level (binary, some college degree or lesser vs college graduate or higher), infertility diagnosis (female factor, male factor, or unexplained), previous in-vitro fertilization (IVF), previous intrauterine insemination (IUI). Pre-pregnancy body mass index (BMI) was calculated as weight (in kilograms) over height (in meters) squared. Infertility diagnosis by a physician was assigned to each patient based on the Society for Assisted Reproductive Technology.
2.5. Statistical Analysis
We present descriptive characteristics of the study population by trimester, and for those women with available measurements at all trimesters. Exposure distributions were evaluated by calculating, for each trimester, the geometric mean and interquartile range concentrations for each chemical biomarker, and by generating correlation matrices within and across trimesters. Phthalates and phenols biomarkers were evaluated as a chemical mixture, first by mutually adjusting for all biomarkers in classical linear regression models. To relax the assumption of linearity in the dose-response associations, in multiple regression all biomarkers concentrations were categorized into quartiles of their distribution and evaluated using the lowest quartile as reference. Trimester-specific analyses were replicated in a set of sensitivity analyses further adjusting for the use of medications, history of polycystic ovary syndrome, length of gestation, and for the total weekly hours of physical activity. As a sensitivity analysis, we also replicated the multiple regression models restricting to women with available measurements at each of the three trimesters (n=154).
Next, we evaluated the mixture outcome-association with Bayesian Kernel Machine Regression (BKMR).23 BKMR flexibly models joint effect of chemicals within a non-parametric framework that allows investigating non-linear dose-responses and interactions within the mixture. For all BKMR analyses, exposure biomarker concentrations were treated as continuous covariates and log-transformed. As a Bayesian technique, BKMR allows providing preliminary knowledge by setting specific values to prior parameters involved in the estimation. However, in our study we used non informative priors for all estimated parameters. Parameters estimation proceeds in an iterative way using the Markov chain Monte Carlo (MCMC) algorithm. We ran 50.000 iterations, and evaluated convergence of the parameters by use of trace plots.23 Convergence was achieved for all parameters after a burn-in phase of 5000 iterations, which were therefore excluded from the analysis (Supplementary Figure 1). The model includes a variable selection procedure, and each biomarker is given a posterior inclusion probability (PIP), representing the relative importance of each exposure within the mixture. We first run different BKMR models for each trimester, evaluating trimester-specific association between EDC mixtures and GWG. Next, to account for the time-varying nature of biomarker concentrations over pregnancy, we used the hierarchical version of BKMR (hBKMR) evaluating all trimesters in the same model. hBKMR operates the variable selection procedure within a hierarchical framework.18 First, the relative importance (group PIP) of each group (trimester) is estimated, representing the relative contribution of the chemical mixture at each time point. Second, within each trimester, the relative importance of each biomarker is calculated (individual PIP). hBKMR assumes that chemical biomarkers within each group do not have interactive effects, and it is recommended in those settings where within-group correlation is high, but between group correlation is low. We presented results from BKMR models by displaying the dose-response relationship for the most important chemical biomarkers, and by displaying the difference in GWG for a change in biomarker concentration between the 10th and 90th percentile. Potential 2-way interactions within chemicals were evaluated, for each pair of exposures that had shown an independent main effect, by plotting the exposure-response function of one exposure at different levels of the second exposure.24 For BKMR analyses we set the other components of the mixture at their median values, and treated biomarker concentrations as continuous predictors, log-transformed due to their pronounced right-skewedness. All analyses were performed using the statistical software R (version 4.0.1).24 When required, statistical tests were two-tailed and p-values below 0.05 were conventionally chosen to indicate statistical significance.
3. RESULTS
The study population consisted of predominantly white and college-educated women who were never smokers (Table 1). The baseline and cycle characteristics (age, BMI, smoking status, race, education, infertility diagnosis, and type of procedure- IUI and IVF) were similar across trimesters. The median number of gestational weeks was 7, 21 and 27 for the first, second and third trimesters, respectively.
Table 1.
Characteristics of the study population over pregnancy
| Trimester 1 (7 gestation weeks) | Trimester 2 (21 gestation weeks) | Trimester 3 (27 gestation weeks) | All trimesters * | |
|---|---|---|---|---|
| N | 240 | 209 | 178 | 154 |
| Age, Mean (SD) | 35.221 (3.777) | 35.110 (3.782) | 35.275 (3.595) | 35.1 (3.6) |
| BMI, Mean (SD) | 24.3 (4.748) | 24.4 (4.937) | 24.1 (4.647) | 24.2 (4.7) |
| Never smokers, n (%) | 183 (76.2%) | 157 (75.1%) | 133 (74.7%) | 113 (73.4%) |
| Race, n (%) | ||||
| Caucasian | 202 (84.2%) | 181 (86.6%) | 153 (86.0%) | 135 (87.7%) |
| Other | 38 (15.8%) | 28 (13.4%) | 25 (14.0%) | 19 (12.3%) |
| Education, n (%) | ||||
| Some college or less | 89 (37.1%) | 78 (37.3%) | 64 (36.0%) | 59 (38.3%) |
| College degree or more | 127 (52.9%) | 110 (52.6%) | 96 (53.9%) | 80 (51.9%) |
| Infertility diagnosis, n (%) | ||||
| Female factor | 62 (25.8%) | 59 (28.2%) | 49 (27.5%) | 42 (27.3%) |
| Male factor | 72 (30.0%) | 61 (29.2%) | 53 (29.8%) | 46 (29.9%) |
| Unexplained | 106 (44.2%) | 89 (42.6%) | 76 (42.7%) | 66 (42.9%) |
| IUI, n (%) | 36 (15.0%) | 30 (14.4%) | 24 (13.5%) | 19 (12.3%) |
| IVF, n (%) | 125 (52.1%) | 106 (50.7%) | 89 (50.0%) | 75 (48.7%) |
indicate individuals with measurements available at all trimesters
Exposure biomarkers distribution and correlation matrices within and across each trimester are presented in Supplementary Tables 1–2, and Supplementary Figure 2. Low to moderate levels of correlation across trimesters were observed (all r<0.5). Within each trimester, high levels of correlations were observed between phthalate metabolites, and between methyl- and propylparaben.
Multiple Linear Regression
The trimester-specific association between GWG and a mixture of EDCs (phthalates, parabens and BPA), mutually adjusted in the same regression model and further adjusting for potential confounders, is presented in Table 2. During 1st trimester, higher GWG was observed at higher ΣDEHP (when comparing 2nd, 3rd, and 4th quartile with the first, respectively: β=1.50 kg, 95% CI: −0.74, 3.73; β=2.17 kg, 95% CI: −0.51, 4.85; β=2.36 kg, 95% CI:−0.47, 5.19). During the 2nd trimester, higher GWG was observed at increasingly higher MBP concentrations (when comparing 2nd, 3rd, and 4th quartile with the first, respectively: β=1.56 kg, 95% CI: −1.13, 4.25; β=2.41 kg, 95% CI: −0.97, 5.78; β=1.48 kg, 95% CI: −2.18, 5.14). Higher 2nd trimester ΣDEHP were instead associated with lowed final GWG (for instance, when comparing the 3rd with the 1st quartile: β=−2.90, 95% CI: −5.72, −0.09). At the 2nd trimester, we also observed a suggestive negative association between methylparaben and GWG, and positive between propylparaben and GWG. Finally, higher final GWG was observed among women with higher 3rd trimester MBP (when comparing 2nd, 3rd, and 4th quartile with the first, respectively: β=1.89 kg, 95% CI: −1.26, 5.04; β=4.05 kg, 95% CI: 0.13, 7.98; β=2.18 kg, 95% CI: −2.44, 6.79). Results were substantially unchanged when replicating these models further adjusting for the use of medications, history of polycystic ovary syndrome, length of gestation, and for the total weekly hours of physical activity (Supplementary Table 3). Negligible differences were also reported when restricting trimester-specific analyses to those women with available exposure biomarker results at each trimester (data not shown).
Table 2.
Trimester-specific associations between urinary phthalates and phenols biomarkers (in μg/L, except for ∑DEHP in μmol/L), evaluated in mutually adjusted linear regression models, and gestational weight gain (in kg)
| Trimester 1 | Trimester 2 | Trimester 3 | ||||
|---|---|---|---|---|---|---|
| Range | Beta (95% CI) | Range | Beta (95% CI) | Range | Beta (95% CI) | |
| BPA | ||||||
| Q1 | (<0.41) | 0 (reference) | (<0.31) | 0 (reference) | (<0.31) | 0 (reference) |
| Q2 | (0.41, 0.90) | 0.09 (−2.24, 2.42) | (0.31, 0.80) | 0.64 (−1.46, 2.74) | (0.31, 0.70) | −0.29 (−2.69, 2.11) |
| Q3 | (0.91, 2.22) | −0.17 (−2.57, 2.23) | (0.81, 1.60) | −0.18 (−2.66, 2.29) | (0.71, 1.20) | −0.76 (−3.40, 1.87) |
| Q4 | (>2.22) | −1.23 (−3.55, 1.08) | (>1.60) | 0.10 (−2.58, 2.78) | (>1.20) | −0.05 (−2.74, 2.65) |
| Methylparaben | ||||||
| Q1 | (<18.06) | 0 (reference) | Q1 (<24.71) | 0 (reference) | Q1 (<24.21) | 0 (reference) |
| Q2 | (18.06, 69.40) | −1.02 (−3.37, 1.33) | (24.71, 75.25) | −0.12 (−3.02, 2.77) | (24.21, 60.0) | 0.13 (−2.25, 2.51) |
| Q3 | (69.41, 295.50) | −1.52 (−4.84, 1.81) | (75.26, 224.25) | −0.92 (−4.44, 2.61) | (60.0, 204.30) | −0.35 (−3.32, 2.62) |
| Q4 | (>295.50) | −2.10 (−6.03, 1.83) | (>224.25) | −3.15 (−7.01, 0.70) | (>204.30) | −2.31 (−6.33, 1.71) |
| Propylparaben | ||||||
| Q1 | (<2.51) | 0 (reference) | (<3.21) | 0 (reference) | (<2.31) | 0 (reference) |
| Q2 | (2.51, 12.90) | −1.05 (−3.35, 1.25) | (3.21, 18.75) | 1.77 (−1.13, 4.67) | (2.31, 10.55) | −0.65 (−3.03, 1.72) |
| Q3 | (12.91, 73.55) | −0.06 (−3.16, 3.05) | (18.76, 66.10) | 2.13 (−1.24, 5.49) | (10.56, 49.12) | −0.06 (−2.86, 2.75) |
| Q4 | (>73.55) | 1.23 (−2.59, 5.05) | (>66.10) | 2.74 (−1.14, 6.61) | (>49.12) | 1.29 (−2.89, 5.46) |
| MBP | ||||||
| Q1 | (<3.41) | 0 (reference) | (<3.61) | 0 (reference) | (<3.43) | 0 (reference) |
| Q2 | (3.41, 8.45) | 0.03 (−2.37, 2.42) | (3.61, 9.00) | 1.56 (−1.13, 4.25) | (3.43, 7.90) | 1.89 (−1.26, 5.04) |
| Q3 | (8.46, 15.90) | 0.30 (−2.70, 3.30) | (9.01, 18.90) | 2.41 (−0.97, 5.78) | (7.91, 16.92) | 4.05 (0.13, 7.98) |
| Q4 | (>15.90) | −0.18 (−3.71, 3.34) | (>18.90) | 1.48 (−2.18, 5.14) | (>16.92) | 2.18 (−2.44, 6.79) |
| MiBP | ||||||
| Q1 | (<2.11) | 0 (reference) | (<2.41) | 0 (reference) | (<2.23) | 0 (reference) |
| Q2 | (2.11, 5.65) | 0.07 (−2.33, 2.48) | (2.41, 5.20) | −0.44 (−2.89, 2.02) | (2.23, 5.40) | 0.81 (−1.77, 3.39) |
| Q3 | (5.66, 11.75) | −0.16 (−2.95, 2.64) | (5.21, 12.00) | 0.63 (−2.29, 3.54) | (5.41, 12.12) | 0.64 (−2.38, 3.66) |
| Q4 | (>11.75) | −0.90 (−3.88, 2.08) | (>12.00) | −0.42 (−3.58, 2.74) | (>12.12) | 0.38 (−2.75, 3.51) |
| MBzP | ||||||
| Q1 | (<0.81) | 0 (reference) | (<0.81) | 0 (reference) | (<0.81) | 0 (reference) |
| Q2 | (0.81, 2.10) | 0.08 (−2.29, 2.46) | (0.81, 2.20) | −0.01 (−2.34, 2.33) | (0.81, 2.05) | −1.08 (−4.00, 1.84) |
| Q3 | (2.11, 5.0) | 1.01 (−1.68, 3.69) | (2.21, 5.61) | 0.30 (−2.38, 2.98) | (2.06, 4.7) | −1.73 (−5.17, 1.71) |
| Q4 | (>5.0) | 1.27 (−1.70, 4.23) | (>5.61) | −0.68 (−3.68, 2.32) | (>4.7) | −0.50 (−4.01, 3.02) |
| ΣDEHP | ||||||
| Q1 | (<0.046) | 0 (reference) | (<0.042) | 0 (reference) | (<0.04) | 0 (reference) |
| Q2 | (0.046, 0.098) | 1.50 (−0.74, 3.73) | (0.042, 0.087) | 0.20 (−2.33, 2.73) | (0.04, 0.074) | −0.54 (−3.07, 1.99) |
| Q3 | (0.099, 0.214) | 2.17 (−0.51, 4.85) | (0.088, 0.177) | −2.90 (−5.72, −0.09) | (0.075, 0.144) | −0.27 (−3.19, 2.64) |
| Q4 | (>0.214) | 2.36 (−0.47, 5.19) | (>0.177) | −0.14 (−3.36, 3.09) | (>0.144) | −0.36 (−3.82, 3.10) |
Bayesian Kernel Machine Regression
We first ran a set of three independent BKMR models, one for each trimester. Results were largely consistent with those obtained from multiple linear regression (data not shown). To evaluate the mixture of EDCs over the pregnancy, we then fit hBKMR incorporating all biomarkers from the 3 time points (trimesters) in the same model. All main conditions for the application of hBKMR to evaluate time-varying mixtures were met, including low levels of correlations between trimesters (Supplementary Table 2), high levels of within-group correlations (Supplementary Figure 2). Table 3 presents the PIPs for each trimester, and for each exposure biomarker within the trimester. The 1st trimester had the highest group PIP, signifying that in our sample this trimester is the most important window of exposure when evaluating prenatal urinary EDCs in relation to final GWG, once all trimesters are considered simultaneously. Within the first trimester, ΣDEHP, MiBP and propylparaben had the highest individual PIPs (0.3, 0.15, 0.15 respectively).
Table 3.
Group (trimester) and individual (exposure) posterior inclusion probability (PIP) from time-varying Bayesian Kernel Machine Regression (BKMR) model
| Biomarker | Trimester PIP | Individual PIP | |
|---|---|---|---|
| Trimester 1 | BPA | 0.51 | 0.09 |
| Methylparaben | 0.51 | 0.09 | |
| Propylparaben | 0.51 | 0.15 | |
| MBP | 0.51 | 0.1 | |
| MiBP | 0.51 | 0.15 | |
| MBzP | 0.51 | 0.12 | |
| ΣDEHP | 0.51 | 0.3 | |
| Trimester 2 | BPA | 0.29 | 0.27 |
| Methylparaben | 0.29 | 0.3 | |
| Propylparaben | 0.29 | 0.14 | |
| MBP | 0.29 | 0.07 | |
| MiBP | 0.29 | 0.09 | |
| MBzP | 0.29 | 0.07 | |
| ΣDEHP | 0.29 | 0.07 | |
| Trimester 3 | BPA | 0.24 | 0.12 |
| Methylparaben | 0.24 | 0.1 | |
| Propylparaben | 0.24 | 0.06 | |
| MBP | 0.24 | 0.35 | |
| MiBP | 0.24 | 0.11 | |
| MBzP | 0.24 | 0.13 | |
| ΣDEHP | 0.24 | 0.13 |
Figure 1 depicts the dose-response relationship between 1st trimester urinary concentrations and final GWG for these three biomarkers, from the hBKMR model. The time-varying analysis confirmed the suggestive positive association between higher 1st trimester ΣDEHP and final GWG. The dose-response associations for all other biomarkers in the mixtures are reported in Supplementary Figure 3, mostly showing null associations.
Figure 1.
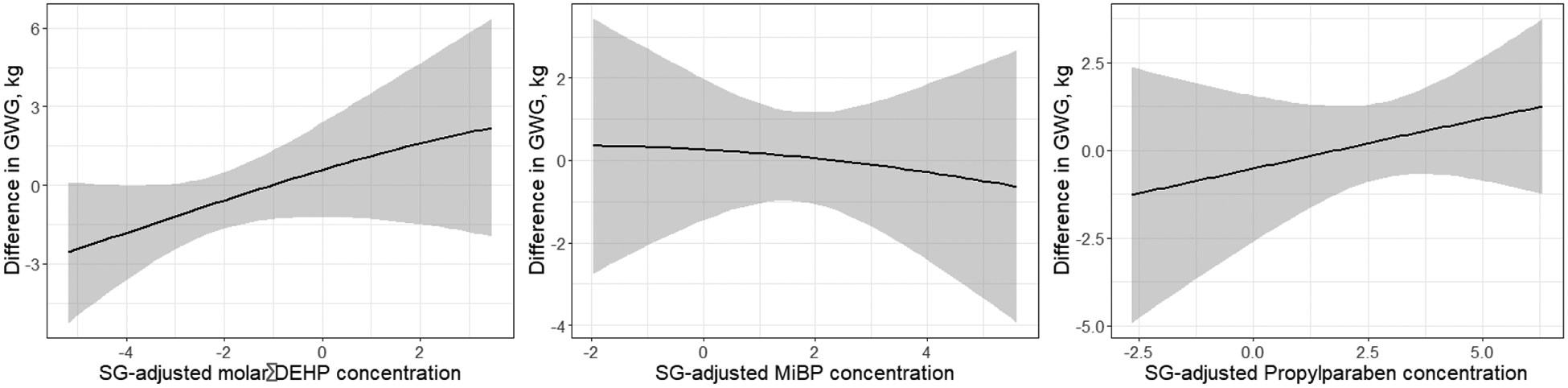
Dose-response relationship between GWG and (a) molar sum of DEHP metabolites, (b) MiBP and (c) propylparaben in the first trimester of pregnancy, obtained from hierarchical Bayesian Kernel Machine Regression models.
In Figure 2, for each trimester, we compare results from the time-varying (hBKMR) and the trimester-specific (BKMR) models. Specifically, we plot the effect of an increase in each exposure biomarker from 10th to 90th percentile, while others are fixed at their median values for each model, on GWG. Results show that the magnitude of the association depends on whether we account for the time-varying nature of the exposures. For example, both ΣDEHP and propylparaben show stronger association in the time-varying model compared to a model that includes only the first trimester, while results for 2nd trimester methylparaben and propylparaben are largely reduced in the time-varying model. No evidence of interaction between these chemicals were detected in this study (Supplementary Figure 4).
Figure 2.
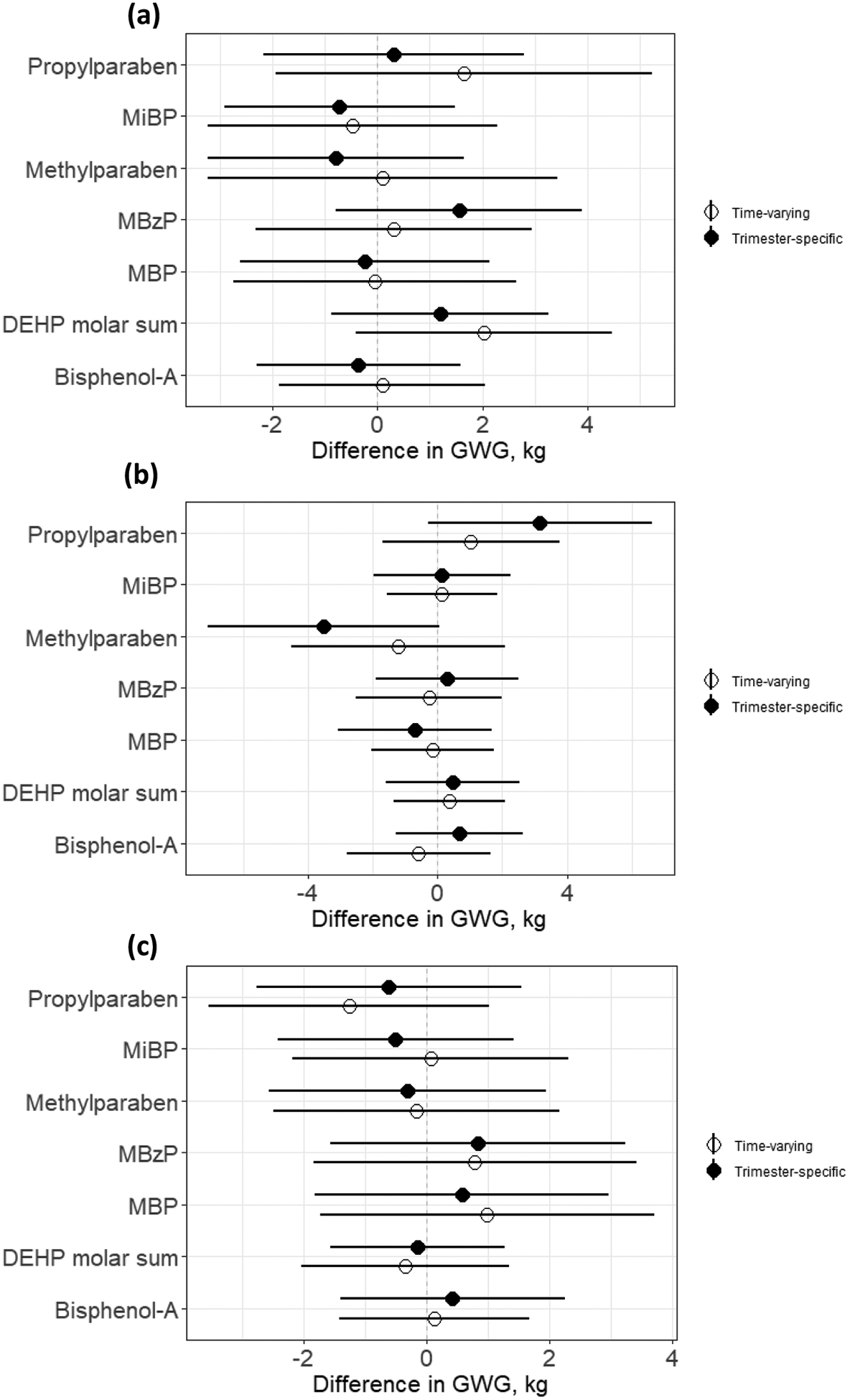
Effect of an increase in each exposure biomarker concentration from 10th to 90th percentile while other exposure biomarkers are fixed at their median values in (a) first, (b) second and (c) third trimesters, from hierarchical (time-varying) Bayesian Kernel Machine Regression models (white dots), and trimester-specific Bayesian Kernel Machine Regression models (black dots)
4. DISCUSSION
In this study we observed suggestive positive associations between EDCs exposure biomarker concentrations during pregnancy, especially DEHP metabolites, and GWG. By accounting for the complex nature of exposures to EDCs as a time-varying mixture of chemicals, our results suggest the 1st trimester of pregnancy as the time window of higher susceptibility to the effects of DEHP as it relates to GWG.
Pregnancy exposure to EDCs has been linked to several adverse health outcomes in both mothers and offspring.11,12,25–30 Recent studies have suggested that endocrine factors such as high levels of leptin and progesterone may lead to the development of excessive GWG,5,6 thus suggesting that EDCs may play a role in developing this condition. To our knowledge, there are only two recent population-based studies that evaluated the association between EDC and GWG. A European-based study of about 1200 women from Generation R evaluated phthalates and phenols in early and mid-pregnancy as they relate to weight gain, mostly finding inconclusive results and only reporting an association between BPA and lower weight gain,12 A second study based on about 600 Chinese women investigated the association between parabens and GWG, suggesting a positive association for parabens at the first trimester.11 None of these studies, however, accounted for the complex nature of the exposure as chemical mixtures.
In our study, both the multiple regression and the BKMR results showed a potential association between 1st trimester DEHP metabolites and final GWG. Phthalates such as DEHP, commonly found in everyday sources of exposure such as plastic, food, and personal care products, may affect weight gain through different complex mechanisms including thyroid and glucose dysregulation.9,31–34 Some phthalates are known to induce the expression of PPAR gamma, modifying the expression of its target genes and the differentiation of these cells into adipocytes.7,8,35 When evaluating trimester-specific association with multiple linear regression models, in addition to observing associations between ∑DEHP and GWG, we also detected a positive association for 2nd and 3rd trimester MBP and suggestive positive associations for 2nd trimester propylparaben and negative for 1st and 2nd trimester methylparaben. The association between MBP and GWG is not confirmed from the BKMR model, which better capture the interrelationships within the mixture. On the other hand, BKMR results seem to support the presence of a negative association with methylparaben and positive with propylparaben. The opposite effects between methylparaben and propylparaben have also been observed in previous studies focusing on other outcomes such as pregnancy glucose levels.27 While possible explanations to these differences have been presented, including the different estrogenic effects and glucocorticoid-like activities between parabens,36–38 future studies should further investigate the properties of parabens and their effects on pregnancy outcomes.
This study was among the first to evaluate pregnancy exposures to EDC as a time-varying mixture, and to use extensions of BKMR to identify whether there are windows of higher susceptibility to the potential endocrine effects of these chemicals. Pregnancy is a state of rapid physiologic changes, and it has been hypothesized that mothers may be particularly susceptible to chemical exposure during certain developmental stage, referred to as windows of susceptibility.14–16 The existence of such windows of susceptibility would be of crucial importance in the context of non-persistent chemicals, as the biological mechanisms activated by the exposure could depend on the actual timing of exposure. Our results suggest the first trimester of pregnancy to be the window of highest susceptibility to EDC exposure, with implications for excessive GWG and its sequelae.
This study has several strengths. First, it is the first study to evaluate pregnancy exposure to several EDCs as a time-varying chemical mixture in relation to GWG, thus addressing the complex nature of exposure and also attempting to identify windows of susceptibility. By using hBKMR, in particular, we could integrate the flexibility of BKMR, which allows to evaluate dose-responses associations in a non-parametric framework that incorporates non-linearities and interactions, as well as retrieve the relative importance of each trimester in generating the mixture-outcome association.18,29 Evaluating the time-varying association of the biomarkers mixture was also possible thanks to the prospective nature of the study, with multiple measurements available for each participant, and with several chemical biomarkers being assessed from urinary samples throughout pregnancy.
This study also has some limitations. First, because of the relatively small sample size, most point estimates showing positive association were accompanied by very large confidence or credible intervals. As such, we cannot completely rule out a chance component in explaining our results. Moreover, because of this relatively small sample, we only looked at GWG as a continuous covariate, without investigating a binary outcome of excessive GWG. The time-varying analysis, in particular, required participants having available measures on all exposure biomarkers at each time point, thus increasing the width of credible intervals. In addition, to maximize the number of individuals with available data on the entire mixture of chemical biomarkers, we could not include several biomarkers with a relatively high number of nondetectable concentrations. Finally, the EARTH study recruits women attending a fertility clinic, which does not necessarily generalize to the entire population.19 Our results should be evaluated in other cohorts, with possibly larger sample sizes, to increase the generalizability of these findings. Furthermore, because of the non-persistent nature of the chemicals analyzed, our findings may be affected by variability within and across trimesters. Future studies where repeated chemicals sampling is available should be conducted to more robustly confirm whether the first trimester is the window of highest susceptibility.
In conclusion, our results agree with prior findings that pregnancy exposure to select EDCs, and especially DEHP, may be involved in dis-regulatory processes leading to adverse health outcomes. Importantly, our results are additionally suggesting that the first trimester of pregnancy may be a time of highest susceptibility to the endocrine disrupting effects of these chemicals, with potential implication for the design of public health interventions and recommendations. Future studies to further evaluate the effects of pregnancy exposures to EDCs and their timing are warranted.
Supplementary Material
HIGHLIGHTS.
Excessive gestational weight gain has harmful implications for mothers and offspring
Environmental factors such as endocrine disruptors may contribute to this condition
Pregnant women may be more susceptible to these exposures in specific time windows
We observed positive associations between EDC mixture and GWG
Results indicate the 1st trimester as the window of highest susceptibility to the EDC effects on GWG
Funding:
This work was supported by National Institutes of Health Grants R01ES026166, R01ES022955, and P30ES000002 from the National Institute of Environmental Health Sciences.
Abbreviations:
- BKMR
Bayesian kernel machine regression
- EDC
endocrine disrupting chemicals
- GWG
gestational weight gain
- IVF
in-vitro fertilization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Conflict of interest: nothing to declare
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM & Lawlor DA Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol 209, 327–e1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva FP et al. Role of Body Mass Index and gestational weight gain on preterm birth and adverse perinatal outcomes. Sci. Rep 9, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein RF et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 317, 2207–2225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention. Gestational Weight Gain — United States, 2012 and 2013. 64(43);1215–1220 (2015) https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6443a3.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lof M et al. Dietary fat intake and gestational weight gain in relation to estradiol and progesterone plasma levels during pregnancy: a longitudinal study in Swedish women. BMC Womens Health 9, 10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacroix M et al. Higher maternal leptin levels at second trimester are associated with subsequent greater gestational weight gain in late pregnancy. BMC Pregnancy Childbirth 16, 62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feige JN et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor γ modulator that promotes adipogenesis. J. Biol. Chem 282, 19152–19166 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Biemann R, Fischer B & Santos AN Adipogenic effects of a combination of the endocrine-disrupting compounds bisphenol A, diethylhexylphthalate, and tributyltin. Obes. Facts 7, 48–56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser R & Calafat AM Phthalates and human health. Occup. Environ. Med 62, 806–818 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen FA Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int. J. Toxicol 27, 1–82 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Wen Q et al. Association between urinary paraben concentrations and gestational weight gain during pregnancy. J. Expo. Sci. Environ. Epidemiol 1–11 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Philips EM et al. Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environ. Int 135, 105342 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aylward LL, Kirman CR, Schoeny R, Portier CJ & Hays SM Evaluation of biomonitoring data from the CDC National Exposure Report in a risk assessment context: perspectives across chemicals. Environ. Health Perspect 121, 287–294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins DJ et al. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ. Res 159, 143–151 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins DJ et al. Impact of phthalate and BPA exposure during in utero windows of susceptibility on reproductive hormones and sexual maturation in peripubertal males. Environ. Health 16, 69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson KK, McElrath TF, Ko Y-A, Mukherjee B & Meeker JD Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ. Int 70, 118–124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu SH et al. Modeling the health effects of time-varying complex environmental mixtures: Mean field variational Bayes for lagged kernel machine regression. Environmetrics 29, e2504 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valeri L et al. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from rural Bangladesh. Environ. Health Perspect 125, 067015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messerlian C et al. The Environment and Reproductive Health (EARTH) Study: a prospective preconception cohort. Hum. Reprod. Open 2018, hoy001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva MJ et al. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B 860, 106–112 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Ye X, Kuklenyik Z, Needham LL & Calafat AM Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal. Chem 77, 5407–5413 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Hornung RW & Reed LD Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5, 46–51 (1990). [Google Scholar]
- 23.Bobb JF et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16, 493–508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobb JF, Henn BC, Valeri L & Coull BA Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 17, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philippat C et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ. Health Perspect 120, 464–470 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W et al. Parabens exposure in early pregnancy and gestational diabetes mellitus. Environ. Int 126, 468–475 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Bellavia A et al. Urinary concentrations of parabens mixture and pregnancy glucose levels among women from a fertility clinic. Environ. Res 168, 389–396 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellavia A et al. Urinary phthalate metabolite concentrations and maternal weight during early pregnancy. Int. J. Hyg. Environ. Health 220, 1347–1355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu Y-H et al. Evaluating effects of prenatal exposure to phthalate mixtures on birth weight: A comparison of three statistical approaches. Environ. Int 113, 231–239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mínguez-Alarcón L et al. Urinary concentrations of bisphenol A, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization. Environ. Int 126, 355–362 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Meeuwen JA, Van Son O, Piersma AH, De Jong PC & Van Den Berg M Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol. Appl. Pharmacol 230, 372–382 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Hu P et al. Effects of parabens on adipocyte differentiation. Toxicol. Sci 131, 56–70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calafat AM, Ye X, Wong L-Y, Bishop AM & Needham LL Urinary concentrations of four parabens in the US population: NHANES 2005–2006. Environ. Health Perspect 118, 679–685 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heindel JJ, Newbold R & Schug TT Endocrine disruptors and obesity. Nat. Rev. Endocrinol 11, 653–661 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Hurst CH & Waxman DJ Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol. Sci 74, 297–308 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Golden R, Gandy J & Vollmer G A review of the endocrine activity of parabens and implications for potential risks to human health. Crit. Rev. Toxicol 35, 435–458 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Aker AM et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ. Res 151, 30–37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taxvig C et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation. Mol. Cell. Endocrinol 361, 106–115 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


