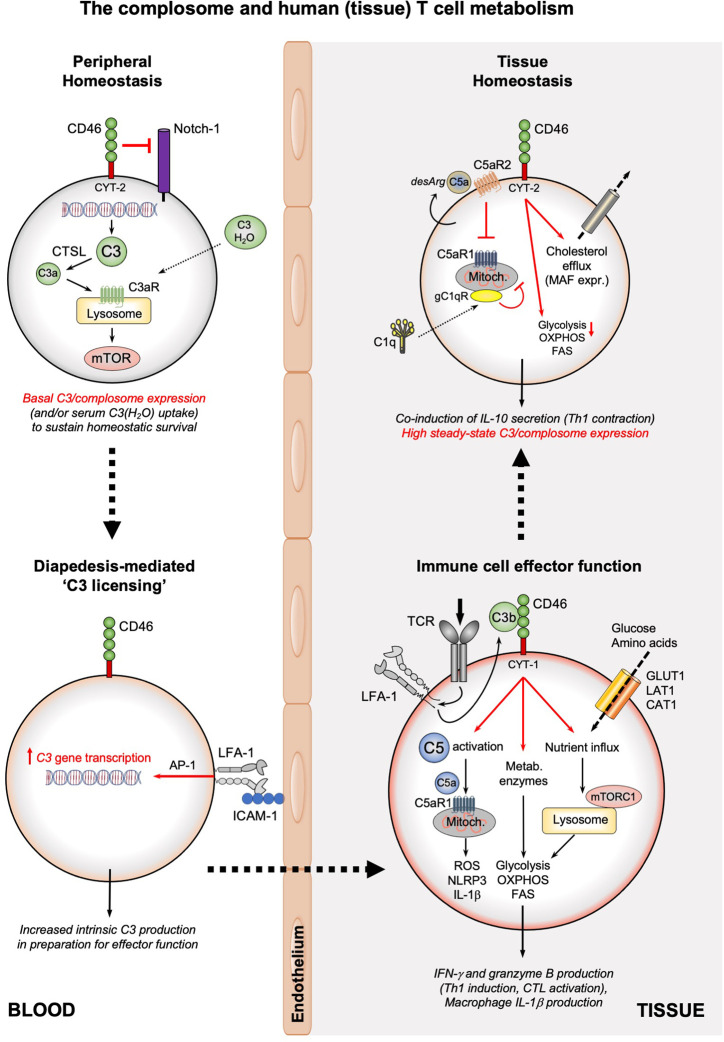
Figure 2.
The complosome and human (tissue) T cell metabolism. The survival of circulating, non-activated CD4+ and CD8+ T cells is maintained by low-level expression of C3 (or uptake of C3(H2O)) that is continuously cleaved by CTSL into intracellular C3a which supports tonic mTOR activation through the lysosomal C3aR. In addition, CD46 surface expression prevents activating Notch-1 stimulation. Diapedesis of T cells (or interaction with APCs presenting cognate antigen, not shown) into tissue involves engagement of LFA-1 on T cells by ICAM-1 on endothelial cells and induces high C3 gene expression in an AP-1-depenent fashion. Timely incoming TCR signals induce rapid translocation of intracellular C3b to the cell surface, where it engages CD46. CD46 signaling triggers three key metabolic events: the γ-secretase-processed intracellular CYT-1 domain of CD46 translocates to the nucleus (not shown) where it induces expression of nutrient transporters (GLUT1, LAT1, and CAT1) as well as LAMTOR5-driven mTORC1 assembly at the lysosomes; CD46 activation induces increased expression of metabolic enzymes, including fatty acid synthase, GAPDH, etc.; CD46 also strongly augments activation of intracellular C5 pools with the intracellularly generated C5a stimulating mitochondrial C5aR1 that drives ROS production and NLRP3 inflammasome activation in CD4+ T cells. Together, these events underly the high levels of glycolysis, OXPHOS and ROS production needed specifically for the induction of IFN-γ production and granzyme B expression and hence protective Th1 and CTL effector responses in tissues. Of note, macrophages also rely on the LFA-1-mediated process for ‘C3 licensing’ to produce normal amounts of IL-1β upon TLR stimulation (not shown). The complosome also contributes to the safe metabolic ‘shut-down’ of human T cell immunity and prevention of tissue pathology as the CD46 intracellular domain CYT-2 reduces glycolysis and OXPHOS while supporting cholesterol efflux and MAF expression, all required for immune-suppressive IL-10 co-induction and demarcating the Th1 contraction phase. This contraction program is further supported by autocrine engagement of the repressive C5aR2 on the T cell surface (via intrinsic C5a-desArg), which reduces C5aR1 activity. C1q, taken up by the activated T cell can reduce mitochondrial activity (in CD8+ T cells) via a C1qR-dependent unknown mechanism. A defining feature of T cells (and macrophages, not shown) in tissues is their high steady-state expression of the complosome. CAT1, cationic amino acid transporter; CTSL, cathepsin L; FAS, fatty acid synthase/synthesis; GLUT1; glucose transporter 1; ICAM-1, intercellular adhesion molecule 1; LFA-1, lymphocyte function-associated antigen 1; LAT1, large neutral amino acid transporter 1; MAF, cMaf musculoaponeurotic fibrosarcoma oncogene homolog; mTOR, mechanistic target of rapamycin; mTORC1, mechanistic target of rapamycin complex 1; NLRP3, NLR family pyrin domain containing 3; OXPHOS, oxidative phosphorylation; ROS, reactive oxidation species; TCR, T cell receptor.