Abstract
Pristine and Co-doped TiO2 mesocrystals have been synthesized via a simple sol–gel method and their antimicrobial activity has been investigated. The antimicrobial performance was evaluated in terms of zone of inhibition, minimum inhibitory concentration (MIC), antibiofilm activity, and effect of UV illumination in liquid media. The Co-doped TiO2 mesocrystals showed very promising MIC of 0.390 μg/mL and 0.781 μg/mL for P. mirabilis and P. mirabilis, respectively. Additionally, the material showed an MIC of 12.5 μg/mL against C. albicans, suggesting its use as antifungal agent. Upon the addition of 10.0 µg/mL of Co-doped TiO2 mesocrystals, the biofilm inhibition% reaches 84.43% for P. aeruginosa, 78.58% for P. mirabilis, and 77.81% for S. typhi, which can be ascribed to the created active oxygen species that decompose the tested microbial cells upon illumination. Thus the fabricated Co-doped TiO2 mesocrystals exhibit sufficient antimicrobial features under visible light, qualifying them for use as antimicrobial agents against pathogenic bacteria and fungi and subsequently inhibit their hazardous effects.
Subject terms: Pathogenesis, Engineering, Nanoscience and technology
Introduction
In the last decades, a lot of efforts have been devoted towards the synthesis of nanomaterials with unique physical, chemical, and biological characteristics compared to their bulk counterparts1,2. Cölfen et al. first introduced a new class of materials known as mesocrystal3. Mesocrystals were proposed to form upon the addition of highly oriented small particles, thus the resulting larger crystals would have single-crystal orientation4. Their positive effects in improving charge carriers separation made them good candidates for many applications, such as photocatalysis5, sensing, and energy storage and conversion6. For instance, TiO2 mesocrystals become a research hotspot for biomedical and food applications due to their antimicrobial characteristics7. Besides, their chemical stability, abundance, low cost, eco-friendly made them good candidates for photovoltaics8, hydrogen production9 and wastewater treatment10. Upon irradiated by UV light, the anatase phase of TiO2 can oxidize and reduce oxygen and water to produce reactive oxygen species (ROS), such as superoxide radicals and hydroxyl radicals11. These ROS play a key role in destroying pathogenic bacteria and fungi by damaging their critical molecular components12,13. However, the practical application of TiO2 photocatalysts is limited by their wide bandgap energy (3.0–3.2 eV), limited to the UV region of the light spectrum with low efficiency of solar light energy utilization14–18. To enhance the photocatalytic response of TiO2, many strategies have been implemented, such as metal and nonmetal doping, annealing in reducing atmosphere, creating defects in the crystal lattice, and coupling with various light harvesters16,19,20. Doping TiO2 with transition metals, such as Fe, Ni, Cr, and Co, was shown to enhance its photoactivity by creating shallow states that suppress the e–h pairs recombination21,22. Moreover, multi-doping with two or three metal or non-metal elements, such as N, C, and Ce or Co, Cu, Ir, C, and Ti3+, was shown to improve the conductivity and balance the deficiencies of individual dopants23,24. Consequently, multi-doped TiO2 has gained much attention compared to the singly and doubly-doped counterparts. The origin of the super reactivity of multi-doped TiO2 seems to be the synergistic effect of the dopants in narrowing the bandgap, enhancing the concentration of reactive radical species25, and enhancing visible light absorption24. In this regard, identifying a one-step synthesis method of multi-doped TiO2 is extremely desirable, which remains a challenge to be realized. Herein, we report on the successful fabrication of cobalt, Ti3+, and carbon multi-doped TiO2 mesocrystals via an in-situ sol–gel process. The antimicrobial behavior of the fabricated mesocrystals was investigated at ambient conditions and under light illumination. Finally, the reaction mechanism of TiO2 and multi-doped TiO2 mesocrystals-treated microbial cells was suggested and discussed in details. Thus, the innovative points of this research include the one-pot synthesis with controlled amount of dopants, the defective structures and how defects played a role in the antibacterial properties as well as the superior dual bacterial and fungi inhibition functions.
Materials and methods
Titanium n-propoxide (Ti(O-n-Pr)4, 98%), cobalt nitrate hexahydrate (Co (NO3)2·6H2O), Formamide (FA: H2N-CHO), and hydrofluoric acid (HF 40%) were purchased from Sigma-Aldrich. Microbiological media ingredients were purchased from Oxford, and reagents used in the biological tests were obtained from Sigma-Aldrich. All the other chemicals were of pure grade and used as received without any further purification. All the solutions were prepared using distilled water (DW).
Synthesis of TiO2 mesocrystals
The proposed TiO2 mesocrystals were fabricated using a facile one-pot synthesis method, inspired by the work reported by Hegazy and Prouzet26. Typically, 3 mL of HF was added dropwise to 4.84 mL of Ti(O-n-Pr)4 with vigorous stirring in an ice bath. Then, 5.2 mL of a solution of FA in DW (86%, v/v) were added dropwise to the previous solution, which was left for 2 h at room temperature. The resulting gel was dried at 100 °C for 4 h and calcined in air at 400 °C for 4 h18. The Co-doped TiO2 mesocrystals were synthesized according to the same procedure by dissolving 0.5 g (Co(NO3)2) in the mixture of FA/DW.
Physicochemical characterizations and antimicrobial activities of TiO2 and Co-doped TiO2 mesocrystals
The crystal properties of the as-synthesized samples were investigated by X-ray diffraction (XRD) patterns recorded on PANalytical X’Pert PRO X-ray diffractometer with Cu Kα radiation (λ = 0.15418 nm, 2θ range = 5°:80°, step size = 0.04°, and scan-step time = 0.5 s). Raman measurements were performed on a Raman microscope (Pro Raman-L Analyzer) with an excitation laser beam wavelength of 532 nm. Fourier transform infrared (FTIR) spectra were recorded on Nicolet 380 Thermo-Scientific in the range of 400–4000 cm−1. The elemental composition was assessed using Thermo-Scientific ESCALAB 250Xi X-ray photoelectron spectroscopy (XPS). The morphological analysis of the as-synthesized nanoparticles was performed using a Zeiss SEM Ultra 60 field-emission scanning electron microscope (FESEM) operating at an accelerating voltage of 5 kV. The nanostructure of the samples was investigated using JOEL JEM-2100 high-resolution transmission electron microscope (HR-TEM) operating at an accelerating voltage of 200 kV; the sample was prepared by dispersing the TiO2 powder in ethanol followed by dropping a small amount on a standard copper TEM grid containing lacy carbon. The UV–Vis absorption spectra of samples were collected using a Shimadzu UV-2600 UV–Vis–NIR spectrophotometer. The photoluminescence spectra (PL) were recorded using Thermo- Scientific LUMINA fluorescence spectrometer.
The antimicrobial potential of as-synthesized TiO2 mesocrystals, Co-doped TiO2 mesocrystals, and Co2+ ions against different pathogenic microbes (yeast and bacteria) are examined via employing the agar-disc diffusion method12. Firstly, the as-synthesized TiO2 mesocrystals, and Co2+ ions are dissolved into distilled water with concentrations 0.01 mg/mL; 10 ppm. The activity of the as-synthesized compounds are examined against different types of bacteria, namely Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Methicillin-resistant Staphylococcus aureus (MRSA), Proteus vulgaris, Salmonella typhi, and Proteus mirabilis. The examined multi-drug resistance bacteria were tested by Vitek two systems (bioMarieux and Marcy-LEtoile, France). Most of them were resistant to antibiotics like Cefapirin, Ciprofloxacin, Amikacin, Norfloxacin, Amoxicillin, Cefoxitin, Gentamicin, Ampicillin, and Cefotaxime. In the microbiological experiments, we performed the biosafety Level-2 (BSL-2). It should be noted that all the inoculums are established and fixed from 2–5 × 108 CFU/mL (0.5 McFarland; at 600 nm). The inhibition of the bacterial growth was defined by the zone of inhibition (ZOI) after 24 h of incubation. Additionally, the antifungal potential of the as-synthesized TiO2 mesocrystals, and Co2+ ions is examined against pathogenic unicellular fungi (Candida albicans and Candida tropicalic). After that, the inoculums of the tested yeast cells are set from 1–4 × 107 CFU/mL. Finally, Nystatin (NS) and Amoxicillin (AX) are conducted as standard antibiotics. AX is similar to penicillin in its bactericidal action against susceptible bacteria during the stage of active multiplication. It acts via the inhibition of cell wall biosynthesis that leads to the death of the bacteria. While, NS is an antifungal that is both fungi-static and fungicidal in vitro against a wide-variety of yeasts and yeast-like fungi. It exerts its antifungal effects via disruption of the fungal cell membrane.
The minimum inhibitory concentrations (MIC) investigation is completed in Luria–Bertani (LB) broth within a serial dilution. Briefly, a positive control (the microorganism and the nutrient), a negative control (the nutrient solely), and the examined TiO2 mesocrystals (beginning with 0.1 mg/mL concentration; 100 ppm) are applied; MIC is defined following 24 h at 37 °C. The inoculums of the tested bacteria are at 3–5 × 108 CFU/mL and 2–3 × 107 CFU/mL to Candida species. MIC is defined by operating ELISA plate (at 600 nm). Finally, the results are statistically analyzed by applying ONE WAY ANOVA, the least significant difference (LSD), and Duncan's multiple ranges, which are calculated by special software (SPSS version 15).
Antibiofilm activities of Co-doped TiO2 mesocrystals
Moreover, a qualitative measurement regarding the biofilm inhibition was defined as stated by G. Christensen et al.27. The noticeable examination of the biofilm which was performed at the tube wall in the absence and presence of the synthesized TiO2 mesocrystals was established. The antibiofilm of the as-synthesized TiO2 mesocrystals (at 10.0 µg/mL) was examined toward the selected bacteria and Candida spp., and was determined and compared with the control (non-treated one). Briefly, 5 mL of the nutrient broth medium was added inside all tubes, and the examined bacteria and yeast were inoculated after adjusted 0.5 McFarland to be 1–2.5 × 108 CFU/mL. After that, they were incubated at 37.0 ± 0.5 °C for 24 h. The contents presented in control and treated tubes were discarded, mixed with Phosphate Buffer Saline (PBS; pH 7.0), and finally desiccated. Then, the bacterial and yeast cells which adhered to the tube walls were fixed with 5 mL sodium acetate (3.0%) for about 15 min, and finally, they were rinsed with de-ionized water. Biofilms which introduced inside tubes were stained with 15 mL Crystal Violet (CV; 0.1%) and washed with de-ionized water to remove the rest of the CV. It must be noted that, for the semi-quantitative antibiofilm estimation, 5 mL of the absolute ethanol was inserted to dissolve the stained bacterial and yeast biofilms28–30. The O.D. of the stained bacterial and yeast biofilms with CV was examined by UV–Vis. spectrophotometer at 570.0 nm. The bacterial and yeast biofilms inhibition percentage was estimated by applying the following relation (Eq. 1) 31:
| 1 |
Effect of UV-irradiation on the antimicrobial abilities of the prepared TiO2 mesocrystals, and Co-doped TiO2 mesocrystals
Furthermore, the antibacterial activity of the as-synthesized TiO2 nanoparticles with and without UV illumination was assessed against the tested pathogenic microbes Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans strains using the optical density method32. The tested microorganisms were stimulated in nutrient broth (NB) overnight at 37 °C. Firstly, 0.5 mL of the overnight culture were inoculated to 5 mL NB tubes that adjusted after 2 h of incubation to standard 0.5 McFarland concentration that standardly equals 1.5 × 108 CFU of bacteria and 0.400 equal (1 × 104 cells/mL) of C. albicans. 100 µL of Co-doped TiO2 mesocrystals were added into the tubes and then incubated at 37 °C for 60 min. While tubes without Co-doped TiO2 mesocrystals were inoculated with bacteria and used as the positive control (subject to UV), tubes without UV illumination were used as the negative control. Typically, 10-W low-pressure mercury lamp was horizontally-placed on the laminar flow and employed as the UV-irradiation source, where 90% of the emitted irradiation was at the specific wavelength (600 nm for bacteria and 630 nm for the fungi). Finally, test tubes were subject to UV-irradiation for 1 h at a distance of about 61 cm. After the incubation, the turbidity of the medium was measured at λ of 600 nm for bacteria and 630 nm for the fungi.
Reaction mechanism using SEM/EDX analysis of TiO2 mesocrystals, and Co-doped TiO2 mesocrystals-treated microbial cells
The sensitive bacterial cells (from the antibiofilm results) were cleaned with Physiological Buffer Saline (PBS) three-times and finally, fixed by 3.5% glutaraldehyde solution. The maintained microbial units were repeatedly-rinsed by PBS and regularly-dried with different concentrations of ethyl alcohol like 30, 50, 70, 90, and 100% for 15 min at 27 ± 2 °C. Following that, the prepared samples were fixed on an aluminum piece regarding SEM/EDX analysis. The morphological features of the control (non-treated P. aeruginosa), TiO2 mesocrystals, and Co-doped TiO2 mesocrystals-treated P. aeruginosa were examined by SEM/EDX investigation.
Results and discussion
Physicochemical characterization
Figure 1a depicts the XRD patterns of both bare and Co-doped TiO2 mesocrystals calcinated at 400 °C. Both samples have tetragonal anatase phase with a space group I41/amd (Ref card No.: 04-014-5762). No diffraction peaks for cobalt or cobalt oxide were detected in the Co-doped TiO2 sample, which may be related to the low cobalt content33. However, the main (1 0 1) diffraction peak is shifted to lower 2 (inset in Fig. 1a), indicating the incorporation of foreign species into the TiO2 lattice, thus changing the Ti4+ local structure34. Besides, it was found that there is a low intensity peak at 26.7° in the diffraction pattern of the bare TiO2 sample, which can be attributed to the main plane (110) of the rutile phase. The intensity of this peak was enhanced after the insertion of cobalt ions as observed in the diffraction pattern of the Co-doped TiO2 sample. The percentage of both anatase and rutile phases was calculated using Eqs. (2) and (3)20:
| 2 |
| 3 |
where IA and IR are the intensities of XRD peaks of anatase and rutile at 25.3° and 26.7° peaks, respectively. It was found that bare TiO2 sample is composed of 99% anatase and only 1% rutile, while the Co-doped TiO2 sample is composed of 93.3% anatase and 6.7% rutile. The increase of rutile% reveals that Co can act as a rutile stabilizer35. Although it is generally accepted for pure phases that anatase exhibits a higher photocatalytic activity compared to rutile TiO2, the existence of very small percent of the rutile phase causes an enhancement in the photocatalytic activity of the samples, even in the case of bare TiO2 mesocrystals, due to the synergistic effects between the two phases compared to pure phases19.
Figure 1.
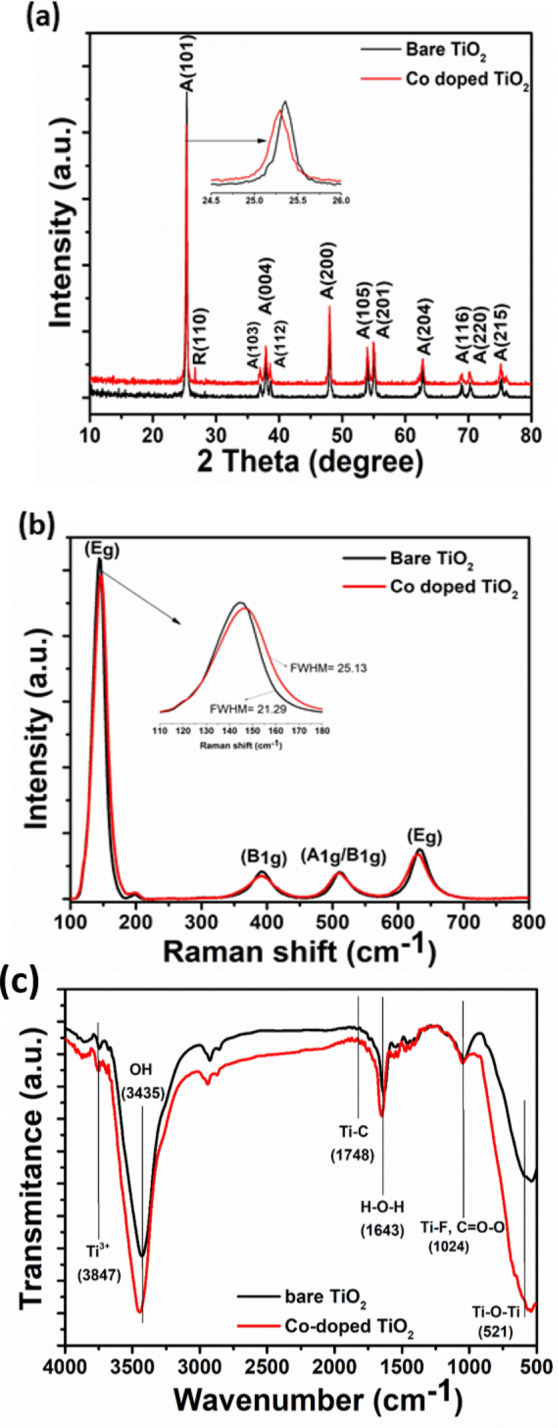
The (a) XRD patterns, (b) Raman, and (c) FT-IR spectra of the bare and Co-doped TiO2 mesocrystals.
The mean crystallite sizes of bare TiO2 and Co-doped TiO2 were estimated to be 69 and 40 nm, respectively as determined using Debey–Scherrer formula (Eq. 4), with L being the mean crystallite size (nm), k the Scherrer constant related to the crystallite shape (k = 0.9), λ the X-ray wavelength in nanometer (nm), β the full width at half-maximum of the peak in radians, and θ the diffraction angle.
| 4 |
Note the decrease in crystallinity upon Co doping, which can be ascribed to the difference between the ionic charge of Ti (+4) and Co (+2). Moreover, Co doping is expected to cause a slight change in the lattice constants. The lattice parameters (a and c) and cell volume (V) were estimated based on Bragg’s law and a formula for a tetragonal system (Eq. 5) for both bare TiO2 and Co-doped TiO2 samples.
| 5 |
It was observed that the unit cell volume has been enlarged from 135.66 to 136.612, and the lattice constant “a” from 3.78 to 3.794 for bare TiO2 and Co-doped TiO2, respectively. These results may indicate the successful incorporation of Co2+ into the anatase TiO2 lattice.
Raman spectroscopy is an effective tool to elucidate the structural changes in materials upon doping. Based on group theory, anatase TiO2 exhibits six Raman-active vibrational modes (3 Eg + 2B1g + A1g), as displayed in Fig. 1b. The six allowed modes of anatase single crystal were reported by Oshaka36, where the bands Eg1 (at 144 cm−1), Eg2 (at 196 cm−1), and Eg3 (at 632 cm−1), for Eg modes are related to the O–Ti–O symmetric stretching vibration. The two B1g modes: B1g1 at 392 and B1g2 at 511 cm−1 are related to the O–Ti–O symmetric bending vibration, and the one A1g mode at 512 cm−1 is related to the O–Ti–O anti-symmetric bending vibration37,38. In the case of Co-doped TiO2 sample, it is found that the main peaks Eg1 and Eg2, located at 144 cm−1 and 196 cm−1, are shifted to higher wavenumbers (147.2 and 199.2 cm−1). However, the Eg3 peak (632 cm−1) is shifted to a lower wavenumber (629.8 cm−1) compared to that of bare TiO2. Besides, the B1g and A1g peaks are shifted to 389.6 and 509.7 cm−1, respectively. Furthermore, no bands appeared related to any cobalt oxide phase, probably due to the low Co content in TiO2 lattice39. As the ionic radius of Co2+ (0.70 Å) is larger than that of Ti4+ (0.64 Å), the insertion of Co as a dopant should lead to a structural distortion and induce oxygen vacancies, which can be the main reason of the observed peak shift. Moreover, electron–phonon coupling is one of the physical parameters used to understand the electron transport and the existence of oxygen vacancies in the lattice of metal oxides20. The electron–phonon coupling is related to the phonon linewidth (FWHM) and can be estimated from the energy-time uncertainty relation (Eq. 6) 40:
| 6 |
where τ is the phonon lifetime, c is the speed of light (3 × 108 m/s), and r is the FWHM of the Raman peak in units of cm−1. The estimated phonon lifetime is found to decrease from 2.49 to 2.11 ps upon cobalt doping.
The FT-IR spectra of the bare and Co-doped TiO2 mesocrystals are shown in Fig. 1c. The broad peaks located at 3847, 3837, and 3800 cm−1 are ascribed to Ti3+41. Besides, the broad peaks at 3435 and 3750 cm−1 are likely due to stretching vibrations of adsorbed O–H groups, while the peak at 1748 cm−1 arises from Ti–O–C vibration, confirming the effective interaction between Ti and C. The peak at 1643 cm−1 is from the H–O–H bending mode42. The peak located at 1427 cm−1 is assigned to the Ti–O vibrations on the {001} facets43, in good agreement with the XRD results. Also, the peak at 1024 cm−1 is due to Ti–F vibrations in both samples. Moreover, the Ti–O stretching and Ti–O–Ti bridging have appeared between 521 and 460 cm−1. Thus, the FT-IR spectra reveals the presence of Ti3+ in the prepared TiO2 mesocrystals.
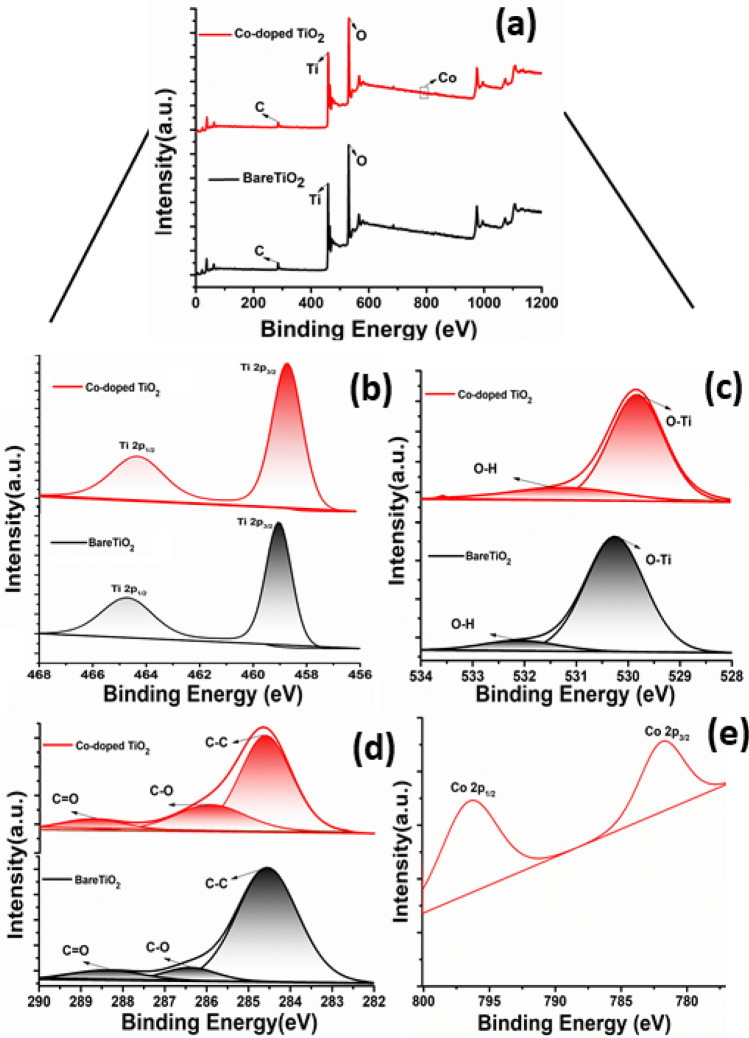
In order to investigate the bonding states of the elements on the surface of bare and Co- doped TiO2, XPS analysis was carried and the data are presented in Fig. 2. The full-scan XPS survey of both samples (Fig. 2a) revealed peaks that are exclusively related to Ti, O, and C, elements in addition to an extra peak of Co in the Co- doped TiO2 sample. Figure 2b shows the high-resolution XPS spectra of Ti 2p, where Ti 2p3/2 and Ti 2p3/2 peaks are observed at 459.1 eV and 464.7 eV, respectively for the bare TiO2 sample. The O1s HR-XPS spectrum (Fig. 2c) is fitted into two-sub peaks centered at 530.26 eV and 532.17 eV for bare TiO2, and 529.78 and 531.4 eV for Co-doped TiO2. The peaks at 530.26 eV and 529.78 eV are related to Ti–O and surface OH-groups44. Additionally, the other oxygen peak (530.3 eV) in Co-doped TiO2 is originated from the presence of Co–O bond45. Moreover, the C 1 s peak (Fig. 2d) can be de-convoluted into three peaks in both samples, one located at 284.69 eV and 284.54 eV and others at 285.82 eV, 286.39 eV, 288.67 eV and 288.42 eV for bare and Co-doped TiO2, respectively. The main peak corresponds to C–C bond that exists in carbon species and the others at higher energy could arise from C–O and C=O bond in TiO2, revealing interstitial and/or substitutional C 46. Finally, the Co 2p peaks appeared at 781.8 and 796.42 eV (Fig. 2e) correspond to Co2+ 2p3/2 and Co2+ 2p1/2, respectively. Notably, all elements binding energies (Ti, O, and C) in Co-doped TiO2 sample exhibited a slight negative shift when compared to the bare TiO2 sample, which can be related to the doping of cobalt ions in TiO2 lattice where the Co has a higher electronegativity than Ti47.
Figure 2.
XPS spectra of bare and Co-doped TiO2mesocrystals: (a) survey spectra, and the HR-spectra of (b) Ti 2p, (c) O 1 s, (d) C 1 s, and (e) Co 2p.
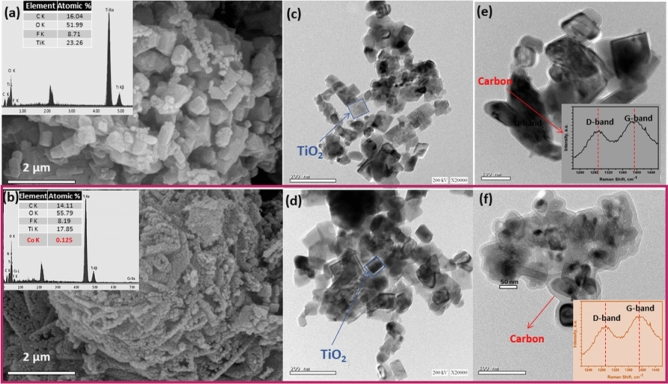
Figure 3a,b shows the representative FESEM images of the prepared undoped and cobalt-doped TiO2 mesocrystals, respectively, revealing agglomerates of highly connected of small particles with homogenous size distribution. Upon Co-doping, a notable decrease in the size of the particles was observed, revealing the effect of cobalt insertion on retarding the TiO2 growth48. The EDX analysis (insets in Fig. 3e,f) reveals the presence of Ti, O, C, and Co without any impurities. Figure 3c–f depicts typical HR-TEM images of the TiO2 mesocrystals before and after doping with cobalt, viewed along a square surface of {001} crystallographic direction. Note also the presence of a carbon shell as indicated by an arrow in Fig. 3e,f. The transparent carbon layer is uniform and continuously surrounding the TiO2 mesocrystals49,50. Raman spectroscopy was used to confirm the presence of the residual carbon (insets in Fig. 3e,f), where the peaks at 1400 and 1290 cm−1 are mainly originating from sp3 hybridization (D-band) and the planar configuration of the sp2-bonded carbon structure (G-band), respectively51.
Figure 3.
(a,b) FESEM images, (a,b insets) EDX analysis, (c–f) HR-TEM images and (e,f insets) Raman spectra of bare and Co-doped TiO2 mesocrystals.
The optical absorption of the bare and Co-doped TiO2 mesocrystals was elucidated by recording their UV–Vis diffuse reflectance spectra (DRS) as shown in Supplementary Fig. S1a. Both samples exhibit visible light response in the wavelength range of 400–600 nm. The spectra of bare TiO2 sample (Supplementary Fig. S1a) reveal an absorption edge at 418 nm, which is redshifted relative to that reported for P25 (TiO2)52. The Co-doped TiO2 sample showed an absorption edge at 424 nm, which is redshifted relative to the bare TiO2. The UV–visible DRS analysis illustrated that bare and Co-doped TiO2 have bandgaps of 2.75 and 2.6 eV, respectively (Supplementary Fig. S1b). The observed redshift in the absorption spectra can be ascribed to Co2+ → Ti4+ charge-transfer53. Thus, photoluminescence (PL) emission spectroscopy was utilized to study the fate of the photoinduced charge carriers54. Oxygen vacancies and surface states play a vital role in the photocatalytic response of anatase TiO255. Supplementary Fig. S1c shows the room-temperature PL spectra of the bare and Co-doped TiO2 samples, where eight peaks started from 396 nm and ended at 700 nm were recorded56. The detected superimposed multi-peaks may reveal radiative recombination of electron–hole pairs from different energy levels57. Notably, the Co-doped TiO2 exhibited a decrease in the PL peaks intensity compared to the bare TiO2, indicating a reduction in the recombination rate of charge carriers in the Co-doped TiO2 sample58. These results suggest the superior photocatalytic activity of Co-doped TiO2 mesocrystals over bare TiO2 mesocrystals.
Antimicrobial properties
Usually antimicrobial agents are used to hinder microbial diseases emanating from clinical poisoning, such as urinary tract infection (UTI)-causing microbes31. However, nanomaterials-based agents have recently received great attention as they are uniquely-applied to combat pathogenic microbes59. In our study, the fabricated samples were checked for their antimicrobial capabilities using the disc agar diffusion technique. The TiO2 samples were found to deactivate a broad spectrum of the tested bacteria such as P. aeruginosa, P. mirabilis, and S. aureus. Specifically, Co-doped TiO2 mesocrsytals showed the most powerful antibacterial effect against all examined microbes, see Fig. 4, Supplementary Fig. S2 and Supplementary Table S1. The antimicrobial abilities of the samples were compared with Co2+ ions and standard antibacterial and antifungal agents like Amoxicillin (AX; 25 μg/mL) and Nystatin (NS; 25 μg/mL). Our samples are found to be more active than the used standard antibiotics, and Co2+ ions. Interestingly, the synthesized TiO2 mesocrystals were found to be more active against Gram-negative bacteria than the Gram-positive counterpart because the cell wall of the Gram-negative bacteria contains a thick layer of lipopolysaccharide essentially in addition to a small layer of peptidoglycan. On contrary, Gram-positive bacteria primarily incorporate a thicker layer of peptidoglycan blocks29. The fabricated NPs enjoy high surface-to-volume ratio, thus can be easily combined and interact with some of the pathogenic microbes, such as yeasts, bacteria, and fungi yeast30.
Figure 4.
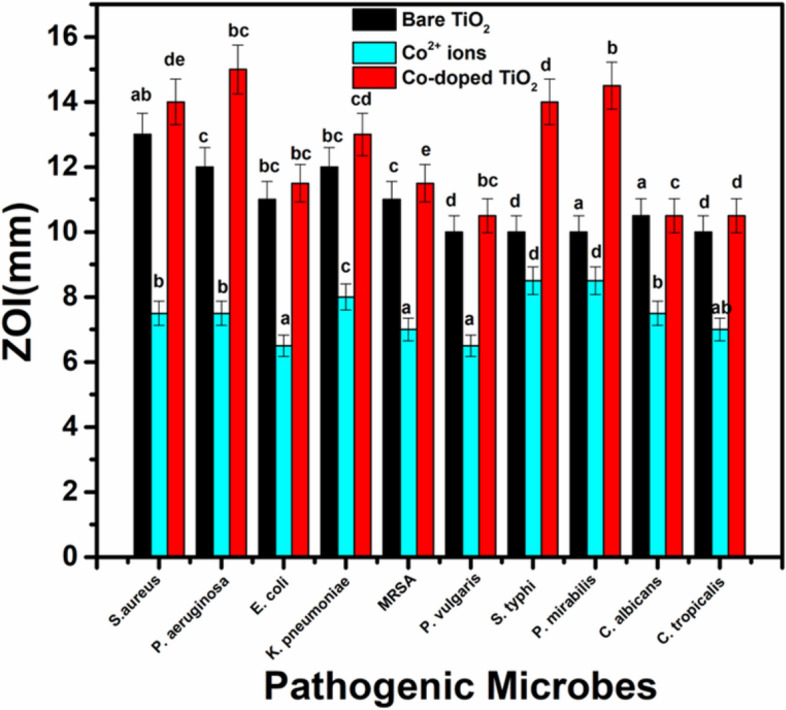
Antimicrobial activity of bare TiO2 NPs and Co-doped TiO2 NPs against different pathogenic microbes as ZIO. The data within the groups are analyzed using a one-way analysis of variance (ANOVA) followed bya,b,c,d,e Duncan’s multiple range test.
The MIC results ranged from 0.39 to 25 μg/mL of the integrated samples against all tested microbes. The promising MIC of the Co-doped TiO2 mesocrystals was 0.39 μg/mL (P. aeruginosa), and 0.781 μg/mL (P. mirabilis). Additionally, the synthesized Co-doped TiO2 NPs exhibit accepted MIC of 12.5 μg/mL against C. albicans at very low NPs concentration (10.0 µg/mL), suggesting their potential use as antifungal agents. Importantly, the properties of the synthesized mesocrystals play a vital role in their antimicrobial characteristics, including their structure, purity, and size51–54. Various advanced mechanisms, such as reactive oxygen species (ROS) distribution (superoxide anion; O2−), were proposed in the literature to explain the possible effects of a plethora of metal oxides as antibacterial agents60–63. However, the antimicrobial mechanism of Co-doped TiO2 mesocrystals has not been identified yet. Thus, the interaction of Co-doped TiO2 mesocrystals with the pathogenic microbes and the alkaline tendency have been included here to demonstrate the possible antimicrobial activity mechanism. It is suggested that Co-doped TiO2 mesocrystals could alter the microbial morphology and their film composition, change the microbial membrane permeability, and produce the residence of oxidative stress genes via the production of H2O260. Note that Co2+ ions were shown to possess antibacterial activity, where a series of Co2+ complexes of mercapto-thiadiazole-derived furanyl, thienyl, pyrrolyl, salicylic, and pyridinyl Schiff bases exhibited in-vitro weak to moderate antibacterial potential toward Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, and Shigella flexneri) and Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureous)64. Another study by Gaëlle et al.65 showed that the ligands, metal salt, and the complexes (Cobalt (II) complex [Co(phen)3(NO3)2]·2H2 O and a novel Co (III) complex) were investigated for their antimicrobial potentials in-vitro toward pathogenic bacteria and fungi. The antimicrobial results indicated that all ligands were very effective towards the tested microbes. The antibacterial activity of pure cobalt or Co2+ ions was attributed to the reaction with negatively-charged molecules inside the microbial cells, which in turn leads to genotoxicity and destruction of the main bacterial organelles31.
Antibiofilm activity of Co-doped TiO2 mesocrystals
The formation of biofilm in pathogenic microbes is characterized by the exo-polysaccharide secretion28,29. The tube method was applied to determine the antibiofilm potential of the synthesized Co-doped TiO2 mesocrystals against some UTI-producing microbes. Supplementary Fig. S3 shows the antibiofilm action of the Co-doped TiO2 mesocrystals for Pseudomonas aeruginosa and Candida albicans. The complete steps are: (I) Normal microbial growth and production of the distinct ring in the lack of the synthesized Co-doped TiO2 mesocrystals and the interference with microbial growth in the closeness of Co-doped TiO2 mesocrystals, (II) The probability of staining of the formed biofilm with Crystal Violet (CV), which is a qualitative determination method, and (III) Eliminating and separating the adhered microbial cells after ethanol addition for semi-quantitative estimation of the biofilm hindrance % (Supplementary Table S2). Supplementary Fig. S3a shows the tube design for the determination of antibiofilm potential of Co-doped TiO2 against P. aeruginosa, the sensitive bacteria example, which creates a thick whitish-yellow layer in the air–liquid interface in the lake of the mesocrystals (control). The produced matt layers were fully-adhered across the walls of the designed tubes and developed as a blue color following the staining with CV. Next, a dark blue color was created in the produced solution subsequent dissolving CV with absolute ethanol, as presented in Supplementary Fig. S3a. On the other side, in the tubes including P. aeruginosa cells and in the closeness of Co-doped TiO2 mesocrystals (10 µg/mL), a remarkable negative effect was recognized as the cells of the tested bacteria do not form biofilm layers and the ring formation was blocked. Also, the adherent cell color was quiet and the blue color was faintly-formed after ethanol addition, as displayed in Supplementary Fig. S3a. Related forms were shown for the biofilm repression of the tested yeast C. albicans as presented in Supplementary Fig. S3b. The semi-quantitative determination of the inhibition percentage (%) was investigated by a UV–visible spectrophotometer. The optical density (O.D.) was estimated at 570 nm following terminating CV-stained biofilms, which were considered as a means of their creation. Supplementary Table S2 displays the inhibition% following the addition of 10.0 µg/mL Co-doped TiO2 mesocrystals, revealing that the highest percentage for P. aeruginosa is 84.43%, for P. mirabilis is 78.58%, and for S. typhi is 77.81%. Note that Co-doped TiO2 mesocrystals were able to control the biofilm growth at its adhesion degree, which is the first step in the antimicrobial process66. The change in the inhibition percentage may be ascribed to many factors such as the high potential of the antimicrobial agents to be attached to the surface due to the high surface area of the synthesized Co-doped TiO2 mesocrystals and their particle size as well as the invasion skills and different chemical characteristics influencing the relationship and communication of Co-doped TiO2 mesocrystals with biofilms-producing microbes67. Positively, the synthesized Co-doped TiO2 mesocrystals suppressed the growth of P. aeruginosa by more than 98% with 0.39 µg/mL MIC as listed in Supplementary Table S2. Figure 5 shows a summarized diagram regarding the antibiofilm potential of Co-doped TiO2 mesocrystals (as inhibition %) against different pathogenic microbes.
Figure 5.
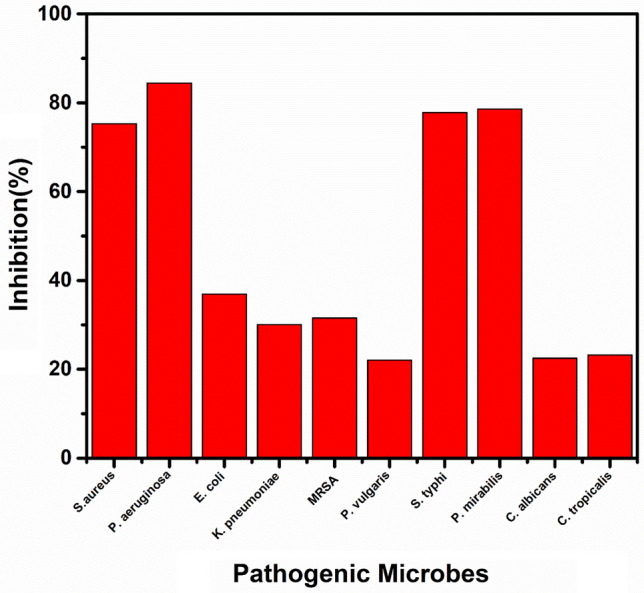
Antibiofilm activity of Co-doped TiO2 against different pathogenic microbes as inhibition %.
Antimicrobial effect of Co-doped TiO2 in liquid media under illumination
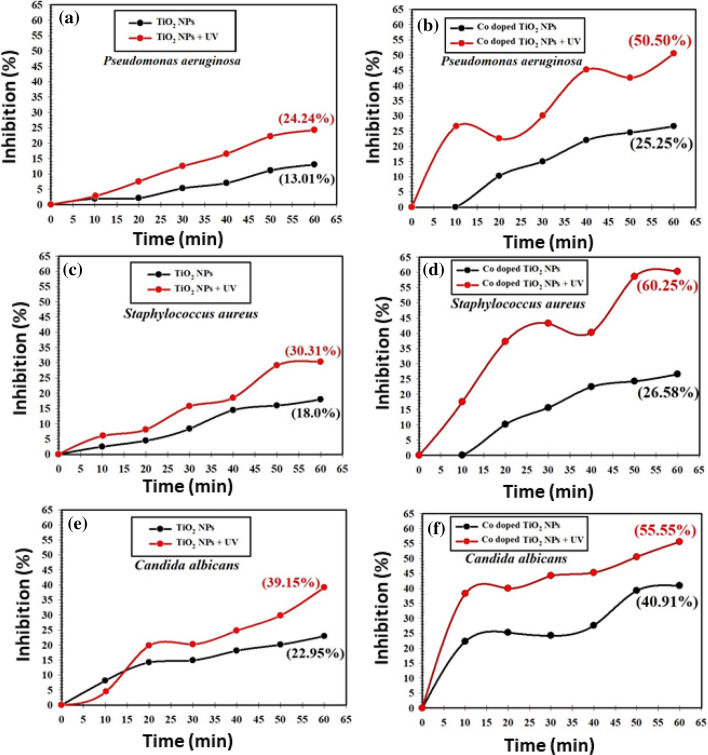
The comparison between the inhibition% of P. aeruginosa, S. aureus, and C. albicans upon the use of TiO2 and Co-doped TiO2 mesocrystals and UV are presented in Fig. 6. Note that Co-doped TiO2 showed higher antimicrobial activities against P. aeruginosa, S. aureus, and C. albicans colonies than pure TiO2, Fig. 6b–d, revealing the synergistic actions of Co doping and the TiO2 mesocrystals. Moreover, upon UV-illumination, Co-doped TiO2 mesocrystals exhibited even higher antimicrobial activities than that in the dark. The maximum inhibition percentage of bare TiO2 and Co-doped TiO2 mesocrystals under UV-illumination for P. aeruginosa at the end of the experiment was 24.24% and 50.50%, respectively (Fig. 6a,b), while it was 30.31% and 60.25% for S. aureus (Fig. 6c,d), and 39.15% and 55.55% for C. albicans (Fig. 6e,f).
Figure 6.
Antimicrobial effect under UV-irradiation effect against different pathogenic microbes, where Pseudomonas aeruginosa (a,b), Staphylococcus aureus (c,d), and Candi1da albicans (e,f), using of bare TiO2, and Co-doped TiO2 in liquid media, respectively.
The observed activity under light irradiation may be related to the induced oxygen species such as OH free radicals, which caused the destruction of the microbial coenzymes and reduced their contents19,42. The major influences involve the creation of holes in the cell wall of the microbes, which subsequently-progressed the cell permeability and finally a cell death will occur. To confirm the induced oxygen species, electron paramagnetic resonance (EPR) spectra were collected as shown in Supplementary Fig. S4. The two samples showed signals at g = 1.95, g = 2.157, g = 2.05, and g = 2.12, confirming the presence of Ti3+ and free oxygen radicals such as OH., O.-, or O2.–68. Notably, the Co-doped TiO2 sample showed a higher concentration of paramagnetic centers of 4.16953 × 1018 spin/g relative to the bare TiO2 sample (4.09473 × 1018 spin/g). The increased paramagnetic centers after the addition of cobalt may be related to the increase in the concentration of oxygen radicals in the TiO6 lattice57. This is in agreement with the inhabitation % of Co-doped versus bare TiO2.
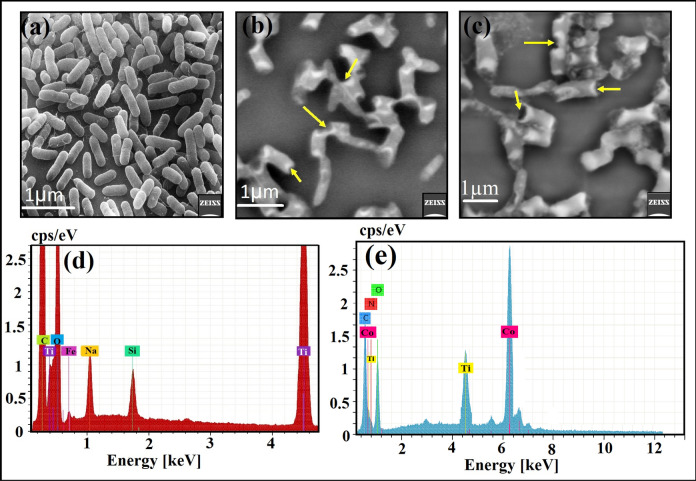
SEM/EDX analysis was performed to elucidate the possible antimicrobial mechanism toward P. aeuroginosa, see Fig. 7. The SEM analysis of the control sample in the absence of any mesocrystals showed bacterial groups that are constantly-developed with typical normal bacterial surface and semi-formed biofilm, Fig. 7a. Upon TiO2 mesocrystals treatment, noticeable morphological differences were identified in P. aeuroginosa (Fig. 7b), including the incomplete lysis of the outer surface followed by deformations of the P. aeuroginosa cells. Additionally, Co-doped TiO2 mesocrystals caused the entire and complete lysis of the bacterial cell with the decrease in the whole viable number, and ultimately the biofilm growth was restrained (Fig. 7c). The EDX elemental analysis shows the presence of Ti and O elements with others from the bacteria like C, and O, along with Fe, Si, and Na form the microelement in the bacterial medium. All detected elements were located at the malformed cities and the outside surface of the P. aeuroginosa cells, validating the performance of the tested TiO2 mesocrystals, (Fig. 7d). Finally, the EDX elemental spectra, in case of Co-doped TiO2, revealed Co, Ti, and O elements along with different atoms from the bacterial structure at the irregular areas and at the outside surface of the treated P. aeuroginosa cells.
Figure 7.
SEM and the matching EDX elemental study of P. aeuroginosa: (a) Regular bacterial cells (P. aeuroginosa) without TiO2 mesocrystals, and Co-doped TiO2 mesocrystals treatment, (b) Abnormal, deformed and irregular bacterial cell with incomplete lysis following TiO2 mesocrystals treatment, (c) Fully-irregular and deformed bacterial cell through Co-doped TiO2 mesocrystals treatment presenting the full lysis of P. aeuroginosa cell, (d) Matching EDX elemental study of the treated P. aeuroginosa cell validating the cellular internalization of the qualified TiO2 mesocrystals in P. aeuroginosa cells, and (e) Matching EDX elemental examination of the treated P. aeuroginosa cell establishing the cellular internalization of the integrated Co-doped TiO2 mesocrystals in P. aeuroginosa cells.
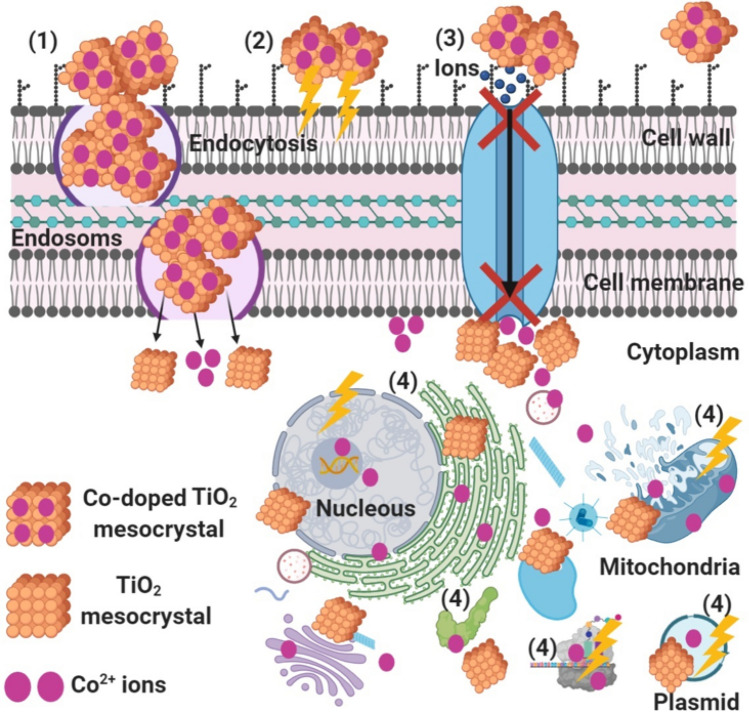
The schematic in Fig. 8 illustrates the possible antibacterial mechanism. We believe that Co-doped TiO2 mesocrystals start their action by adhesion at the outer surface of the bacterial cell, causing membrane damage and altered transport activity. Then, diffusion of Co2+ inside the bacterial cell (at pH = 3) and dividing all of the intracellular structure like mitochondria, plasmid, DNA, and other vital organelles. Afterwards, cellular toxicity occurs due to the oxidative stress generated by the production of ROS. Finally, TiO2 mesocrystals were withstood the acidic condition inside the bacterial cells and conversion did not occur69 but possessed the antibacterial effect by affecting the signal transduction pathways.
Figure 8.
Schematic representation regarding the four prominent ways of antimicrobial potential of Co-doped TiO2 mesocrystals, where (1) Co-doped TiO2 mesocrystals adhere to the bacterial cell surface and results in membrane damage and altered transport activity; (2) Co-doped TiO2 mesocrystals create and increase the ROS leading to cell damage, (3) Co-doped TiO2 mesocrystals block the ions transport from and to the bacterial cell, and (4) Co-doped TiO2 mesocrystals penetrate inside the bacterial cells and interact with cellular organelles and biomolecules, and thereby, affect respective cellular machinery, and modulate the cellular signal system and causing cell death. Co-doped TiO2 mesocrystals may serve as a vehicle to effectively-deliver Co ions to the bacterial cytoplasm and membrane, where proton motive force would decrease the pH to be less than 3.0 and therefore improve the release of Co ions.
Conclusion
In summary, unique multi-doped TiO2 mesocrystals have been synthesized via a facile sol–gel approach. The crystal structure, optical, and compositional properties of the materials were elucidated using XRD, Raman, FTIR, XPS UV–vis analyses. The synthesized Co-doped TiO2 mesocrystals showed excellent antimicrobial activity compared to bare TiO2 counterparts. The antimicrobial performance was evaluated in terms of zone of inhibition, minimum inhibitory concentration (MIC), antibiofilm activity, and photoactivity. Co-doped TiO2 mesocrystals showed 60.25% inactivation of S. aureus after 60 min of UV illumination. The SEM findings supported the results of the viability tests, demonstrating complete lysis of the bacterial cells with the decrease in the whole viable number. The Co-doped TiO2 mesocrystals showed very promising MIC of 0.390 μg/mL and 0.781 μg/mL for P. mirabilis and P. mirabilis, respectively. Additionally, the material showed an MIC of 12.5 μg/mL against C. albicans, suggesting its use as antifungal agent. Considering the efficient fast and oxidative damage mediated inactivation of bacteria on the Co-doped TiO2 mesocrystals showed in this study, our results supports further development and application of Co-doped TiO2 mesocrystals in other fields.
Supplementary Information
Acknowledgements
We acknowledge BioRender for posting the tools freely of charge to allow the creation of professional science figures in minutes that we have used to create Fig. 8.
Author contributions
A.N.E., A.H.H., and M.A.H. synthesized and characterized the mesocrystals, and analyzed the results. G.S.E. and R.M.F. performed the antimicrobial experiments and analyzed the results. N.K.A. analyzed the results and coordinated the project. All authors contributed to the writing of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ayat N. El-Shazly, Gharieb S. El-Sayyad and Aiat H. Hegazy.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84989-x.
References
- 1.Navya PN, Daima HK. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016;3:1–14. doi: 10.1186/s40580-016-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbas W, Ali BA, Abdullah IM, Ahmed N, Allam NK. Recent advances in the use of TiO2 nanotubes powder in biological, environmental, and energy applications. Nanoscale Adv. 2019;1:2801–2816. doi: 10.1039/C9NA00339H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cölfen H, Antonietti M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chemie Int. Ed. 2005;44:5576–5591. doi: 10.1002/anie.200500496. [DOI] [PubMed] [Google Scholar]
- 4.Sturm EV, Cölfen H. Mesocrystals: Structural and morphogenetic aspects. Chem. Soc. Rev. 2016;45:5821–5833. doi: 10.1039/C6CS00208K. [DOI] [PubMed] [Google Scholar]
- 5.Yao X, Hu X, Liu Y, Wang X, Hong X, Chen X, Pillai CX, Dionysiou DD, Wang D. Simultaneous photocatalytic degradation of ibuprofen and H2 evolution over Au/sheaf-like TiO2 mesocrystals. Chemosphere. 2020;261:127759. doi: 10.1016/j.chemosphere.2020.127759. [DOI] [PubMed] [Google Scholar]
- 6.Samir M, Ahmed N, Ramadan M, Allam NK. Electrospun mesoporous Mn-V-O@C nanofibers for high performance asymmetric supercapacitor devices with high stability. ACS Sustain. Chem. Eng. 2019;7:13471–13480. doi: 10.1021/acssuschemeng.9b03026. [DOI] [Google Scholar]
- 7.Chen Y-F, Tang X-N, Zhang B, Luo Y, Li Y. TiO2@SiO2 composites: Preparation and photocatalytic antimicrobial performance. J. Inorg. Mater. 2019;34:1325–1333. doi: 10.15541/jim20190039. [DOI] [Google Scholar]
- 8.Hegazy AH, Kinadjian N, Sadeghimakki B, Sivoththaman S, Allam NK, Prouzet E. TiO2 nanoparticles optimized for photoanodes tested in large area dye-sensitized solar cells (DSSC) Sol. Energy Mater. Sol. Cells. 2016;153:108–116. doi: 10.1016/j.solmat.2016.04.004. [DOI] [Google Scholar]
- 9.Ahmed N, Ramadan M, Farghali AA, El Rouby WMA, Allam NK. Non-precious co-catalysts boost the performance of TiO2 hierarchical hollow mesoporous spheres in solar fuel cells. Int. J. Hydrogen Energy. 2018;43:21219–21230. doi: 10.1016/j.ijhydene.2018.10.012. [DOI] [Google Scholar]
- 10.Ismael AM, El-Shazly AN, Gaber SE, Rashad MM, Kamel AH, Hassan SSM. Novel TiO2/GO/CuFe2O4 nanocomposite: A magnetic, reusable and visible-light-driven photocatalyst for efficient photocatalytic removal of chlorinated pesticides from wastewater. RSC Adv. 2020;10:34806–34814. doi: 10.1039/D0RA02874F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden SC, Allam NK, El-Sayed MA. TiO2 nanotubes/CdS hybrid electrodes:Extraordinarily enhancement in the inactivation of Escherichia coli. J. Am. Chem. Soc. 2010;132:14406–14408. doi: 10.1021/ja107034z. [DOI] [PubMed] [Google Scholar]
- 12.Vatansever F, de Melo CMA, Avci P, Vecchio D, Sadasivam M, Gupta A, Chandran R, Karimi M, Parizotto NA, Yin R, Tegos GP, Hamblin MR. Antimicrobial strategies centered around reactive oxygen species—bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia P, Cao S, Zhu B, Liu M, Shi M, Yu J, Zhang Y. Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria. Angew. Chem. Int. Ed. 2020;59:5218–5225. doi: 10.1002/anie.201916012. [DOI] [PubMed] [Google Scholar]
- 14.Abdelhafiz AA, Ganzoury MA, Amer AW, Faiad AA, Khalifa AM, AlQaradawi AY, El-Sayed MA, Alamgir FM, Allam NK. Defect engineering in 1D Ti-W oxide nanotube arrays and their correlated photoelectrochemical performance. Phys. Chem. Chem. Phys. 2018;20:10258–10265. doi: 10.1039/C8CP01413B. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim EM, Hasan MM, Saleh AA, Allam NK. Structural engineering of Ti–Mn bimetallic phosphide nanotubes for efficient photoelectrochemical water splitting. Int. J. Hydrogen Energy. 2021;46:3605–3614. doi: 10.1016/j.ijhydene.2020.10.262. [DOI] [Google Scholar]
- 16.Deyab NM, Salem KE, Mokhtar AM, Ramadan M, Allam NK. Electrochemical fabrication of ternary black Ti–Mo–Ni oxide nanotube arrays for enhanced photoelectrochemical water oxidation. ChemistrySelect. 2020;5:12151–12158. doi: 10.1002/slct.202003491. [DOI] [Google Scholar]
- 17.El-Shazly AN, Hegazy AH, Rashad MM, El-Shahat MF, Allam NK. Ultrathin ALD TiO2 shells for enhanced photoelectrochemical solar fuel generation. J. Alloys Compd. 2018;739:178–183. doi: 10.1016/j.jallcom.2017.12.218. [DOI] [Google Scholar]
- 18.El-shazly AN, Hegazy AH, El Shenawy ET, Hamza MA, Allam K. Solar energy materials and solar cells novel facet-engineered multi-doped TiO2 mesocrystals with unprecedented visible light photocatalytic hydrogen production. Sol. Energy Mater. Sol. Cells. 2021;220:110825. doi: 10.1016/j.solmat.2020.110825. [DOI] [Google Scholar]
- 19.Fawzy SM, Omar MA, Allam NK. Photoelectrochemical water splitting by defects in nanostructured multinary transition metal oxides. Sol. Energy Mater. Sol. Cells. 2019;194:184–194. doi: 10.1016/j.solmat.2019.02.011. [DOI] [Google Scholar]
- 20.Soliman MM, Al Haron MM, Samir M, Tolba SA, Shaheen BS, Amer AW, Mohammed OF, Allam NK. On the relationship between rutile/anatase ratio and the nature of defect states in sub-100 nm TiO2 nanostructures: Experimental insights. Phys. Chem. Chem. Phys. 2018;20:5975–5982. doi: 10.1039/C7CP08629F. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Guo Z, He T. The doping mechanism of Cr into TiO2 and its influence on the photocatalytic performance. Phys. Chem. Chem. Phys. 2013;15:20037–20045. doi: 10.1039/c3cp53531b. [DOI] [PubMed] [Google Scholar]
- 22.Gao B, Wang W, Fan X, Gong H, Guo H, Xia W, Feng Y, Huang X, He J. Synthesis of yellow mesoporous Ni-doped TiO2 with enhanced photoelectrochemical performance under visible light. Inorg. Chem. Front. 2017;4:898–906. doi: 10.1039/C6QI00609D. [DOI] [Google Scholar]
- 23.Zhao XG, Huang LQ. Iridium, carbon and nitrogen multiple-doped TiO2 nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2017;43:3975–3980. doi: 10.1016/j.ceramint.2016.11.083. [DOI] [Google Scholar]
- 24.Charanpahari A, Umare SS, Sasikala R. Effect of Ce, N and S multi-doping on the photocatalytic activity of TiO2. Appl. Surf. Sci. 2013;282:408–414. doi: 10.1016/j.apsusc.2013.05.144. [DOI] [Google Scholar]
- 25.Wang R, Shi M, Xu F, Qiu Y, Zhang P, Shen K, Zhao Q, Yu J, Zhang Y. Graphdiyne-modified TiO2 nanofibers with enhanced photocatalytic antibacterial and osteoinductive activities for implant infection. Nat. Commun. 2020;11:4465. doi: 10.1038/s41467-020-18267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegazy A, Prouzet E. Room temperature synthesis and thermal evolution of porous nanocrystalline TiO2 anatase. Chem. Mater. 2012;24:245–254. doi: 10.1021/cm201602a. [DOI] [Google Scholar]
- 27.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 1982;37:318–326. doi: 10.1128/IAI.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansari MA, Khan HM, Khan AA, Cameotra SS, Pal R. Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum β-lactamase isolates of Escherichia coli and Klebsiella pneumoniae. Appl. Nanosci. 2014;4:859–868. doi: 10.1007/s13204-013-0266-1. [DOI] [Google Scholar]
- 29.Maksoud MIAA, El-Sayyad GS, Ashour AH, El-Batal AI, Elsayed MA, Gobara M, El-Khawaga AM, Abdel-Khalek EK, El-Okr MM. Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microb. Pathog. 2019;127:144–158. doi: 10.1016/j.micpath.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 30.El-Batal AI, El-Sayyad GS, Al-Hazmi NE, Gobara M. Antibiofilm and antimicrobial activities of silver boron nanoparticles synthesized by PVP polymer and gamma rays against urinary tract pathogens. J. Clust. Sci. 2019;30:947–964. doi: 10.1007/s10876-019-01553-4. [DOI] [Google Scholar]
- 31.Abd Elkodous M, El-Sayyad GS, Abdelrahman IY, El-Bastawisy HS, Mohamed A, Mosallam FM, Nasser HA, Gobara M, Baraka A, Elsayed MA, El-Batal AI. Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surfaces B Biointerfaces. 2019;180:411–428. doi: 10.1016/j.colsurfb.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 32.El-Nemr KF, Mohamed HR, Ali MA, Fathy RM, Dhmees AS. Polyvinyl alcohol/gelatin irradiated blends filled by lignin as green filler for antimicrobial packaging materials. Int. J. Environ. Anal. Chem. 2019 doi: 10.1080/03067319.2019.1657108. [DOI] [Google Scholar]
- 33.Chen WF, Koshy P, Huang Y, Adabifiroozjaei E, Yao Y, Sorrell CC. Effects of precipitation, liquid formation, and intervalence charge transfer on the properties and photocatalytic performance of cobalt- or vanadium-doped TiO2 thin films. Int. J. Hydrogen Energy. 2016 doi: 10.1016/j.ijhydene.2016.08.115. [DOI] [Google Scholar]
- 34.Niu H, Wang Q, Liang H, Chen M, Mao C, Song J, Zhang S, Gao Y, Chen C. Visible-light active and magnetically recyclable nanocomposites for the degradation of organic dye. Materials (Basel). 2014;7:4034–4044. doi: 10.3390/ma7054034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lontio Fomekong R, Saruhan B. Synthesis of Co3+ doped TiO2 by co-precipitation route and its gas sensing properties. Front. Mater. 2019;6:1–6. doi: 10.3389/fmats.2019.00252. [DOI] [Google Scholar]
- 36.Ohsaka T. Temperature dependence of the Raman spectrum in anatase TiO2. J. Phys. Soc. Jpn. 1980;48:1661–1668. doi: 10.1143/JPSJ.48.1661. [DOI] [Google Scholar]
- 37.Samir M, Salama M, Allam NK. Sub-100 nm TiO2 tubular architectures for efficient solar energy conversion. J. Mater. Chem. A. 2016;4:9375–93805. doi: 10.1039/C6TA03156K. [DOI] [Google Scholar]
- 38.Saleh AA, Farag M, Allam NK. Correlation between microstructural defects and photoelectrochemical performance of one-dimensional Ti-Nb composite oxide photoanodes for solar water splitting. Int. J. Hydrogen Energy. 2019;44:24418–24429. doi: 10.1016/j.ijhydene.2019.07.219. [DOI] [Google Scholar]
- 39.Shao X, Lu W, Zhang R, Pan F. Enhanced photocatalytic activity of TiO2-C hybrid aerogels for methylene blue degradation. Sci. Rep. 2013;3:1–9. doi: 10.1038/srep03018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faid AF, Allam NK. Stable solar-driven water splitting by anodic ZnO nanotubular semiconducting photoanodes. RSC Adv. 2016;6:80221–80225. doi: 10.1039/C6RA18747A. [DOI] [Google Scholar]
- 41.Samsudin EM, Abd Hamid SB, Juan JC, Basirun WJ, Centi G. Synergetic effects in novel hydrogenated F-doped TiO2 photocatalysts. Appl. Surf. Sci. 2016;370:380–393. doi: 10.1016/j.apsusc.2016.02.172. [DOI] [Google Scholar]
- 42.El-Sayed A, Atef N, Hegazy AH, Mahmoud KR, AbdelHameed RM, Allam NK. Defect states determined the performance of dopant-free anatase nanocrystals in solar fuel cells. Sol. Energy. 2017;144:445–452. doi: 10.1016/j.solener.2017.01.056. [DOI] [Google Scholar]
- 43.Zhang Y, Zhang Q, Xia T, Zhu D, Chen Y, Chen X. The influence of reaction temperature on the formation and photocatalytic hydrogen generation of (001) faceted TiO2 nanosheets. ChemNanoMat. 2015;1:270–275. doi: 10.1002/cnma.201500030. [DOI] [Google Scholar]
- 44.Kumaravel V, Rhatigan S, Mathew S, Bartlett J, Nolan M, Hinder SJ, Sharma PK, Singh A, Byrne JA, Harrison J, Pillai SC. Indium-doped TiO2 photocatalysts with high-temperature anatase stability. J. Phys. Chem. C. 2019;123:21083–21096. doi: 10.1021/acs.jpcc.9b06811. [DOI] [Google Scholar]
- 45.Akshay VR, Arun B, Mandal G, Mutta GR, Chanda A, Vasundhara M. Observation of optical band-gap narrowing and enhanced magnetic moment in co-doped sol-gel-derived anatase TiO2 nanocrystals. J. Phys. Chem. C. 2018;122:26592–26604. doi: 10.1021/acs.jpcc.8b06646. [DOI] [Google Scholar]
- 46.Liu G, Han C, Pelaez M, Zhu D, Liao S, Likodimos V, Ioannidis N, Kontos AG, Falaras P, Dunlop PSM, Byrne A, Dionysiou DD. Synthesis, characterization and photocatalytic evaluation of visible light activated C-doped TiO2 nanoparticles. Nanotechnology. 2012;23:294003. doi: 10.1088/0957-4484/23/29/294003. [DOI] [PubMed] [Google Scholar]
- 47.Liu C, Wang F, Zhu S, Xu Y, Liang Q, Chen Z. Controlled charge-dynamics in cobalt-doped TiO2 nanowire photoanodes for enhanced photoelectrochemical water splitting. J. Colloid Interface Sci. 2018;530:403–411. doi: 10.1016/j.jcis.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Siddiqa A, Masih D, Anjum D, Siddiq M. Cobalt and sulfur co-doped nano-size TiO2 for photodegradation of various dyes and phenol. J. Environ. Sci. 2015;37:100–109. doi: 10.1016/j.jes.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 49.Hasan M, Tolba S, Allam NK. In-situ formation of graphene stabilizes the zero-valent copper nanoparticles and significantly enhances the efficiency of photocatalytic water splitting. ACS Sustain. Chem. Eng. 2018;6:16876–16885. doi: 10.1021/acssuschemeng.8b04219. [DOI] [Google Scholar]
- 50.Vasei M, Das P, Cherfouth H, Marsan B, Claverie JP. TiO2@C core-shell nanoparticles formed by polymeric nano-encapsulation. Front. Chem. 2014;2:1–9. doi: 10.3389/fchem.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schito, A. & Corrado, S. An automatic approach for characterization of the thermal maturity of dispersed organic matter Raman spectra at low diagenetic stages. Geol. Soc. London, Spec. Publ.484, 107–119 (2018).
- 52.Mai W, Wen F, Xie D, Leng Y, Mu Z. Structure and composition study of carbon-doped titanium oxide film combined with first principles. J. Adv. Ceram. 2014;3:49–55. doi: 10.1007/s40145-014-0092-2. [DOI] [Google Scholar]
- 53.Khan MA, Al-Oufi M, Tossef A, Al-Salik Y, Idriss H. On the role of CoO in CoOx/TiO2 for the photocatalytic hydrogen production from water in the presence of glycerol. Catal. Struct. React. 2015;1:192–200. doi: 10.1080/2055074X.2015.1124191. [DOI] [Google Scholar]
- 54.Yu JG, Yu HG, Cheng B, Zhao XJ, Yu JC, Ho HK. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B. 2003;107:13871–13879. doi: 10.1021/jp036158y. [DOI] [Google Scholar]
- 55.Khan H, Swati IK. Fe3+-doped anatase TiO2 with d-d transition, oxygen vacancies and Ti3+ centers: Synthesis, characterization, UV-vis photocatalytic and mechanistic studies. Ind. Eng. Chem. Res. 2016;55:6619–6633. doi: 10.1021/acs.iecr.6b01104. [DOI] [Google Scholar]
- 56.Pan S, Liu X, Guo M, Yu SF, Huang H, Fan H, Li G. Engineering the intermediate band states in amorphous Ti3+-doped TiO2 for hybrid dye-sensitized solar cell applications. J. Mater. Chem. A. 2015;3:11437–11443. doi: 10.1039/C5TA00956A. [DOI] [Google Scholar]
- 57.Su CH, Hu CC, Sun YCC, Hsiao YC. Highly active and thermo-stable anatase TiO2 photocatalysts synthesized by a microwave-assisted hydrothermal method. J. Taiwan Inst. Chem. Eng. 2016;59:229–236. doi: 10.1016/j.jtice.2015.07.029. [DOI] [Google Scholar]
- 58.Hashem EM, Hamza MA, El-Shazly AN, Abd El-Rahman SA, El-Tanany EM, Mohamed RT, Allam NK. Novel Z-scheme/type-II CdS@ZnO/g-C3N4 ternary nanocomposites for the durable photodegradation of organics: Kinetic and mechanistic insights. Chemosphere. 2020 doi: 10.1016/j.chemosphere.2020.128730. [DOI] [PubMed] [Google Scholar]
- 59.Karthik K, Dhanuskodi S, Gobinath C, Prabukumar S, Sivaramakrishnan S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol. B Biol. 2019;190:8–20. doi: 10.1016/j.jphotobiol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 60.El-Batal AI, Al-Hazmi NE, Mosallam FM, El-Sayyad GS. Biogenic synthesis of copper nanoparticles by natural polysaccharides and Pleurotus ostreatus fermented fenugreek using gamma rays with antioxidant and antimicrobial potential towards some wound pathogens. Microb. Pathog. 2018;118:159–169. doi: 10.1016/j.micpath.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Mosallam FM, El-Sayyad GS, Fathy RM, El-Batal AI. Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microb. Pathog. 2018;122:108–116. doi: 10.1016/j.micpath.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 62.Tang ZX, Lv BF. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014;31:591–601. doi: 10.1590/0104-6632.20140313s00002813. [DOI] [Google Scholar]
- 63.Karthik K, Shashank M, Revathi V, Tatarchuk T. Facile microwave-assisted green synthesis of NiO nanoparticles from Andrographis paniculata leaf extract and evaluation of their photocatalytic and anticancer activities. Mol. Cryst. Liq. Cryst. 2018;673:70–80. doi: 10.1080/15421406.2019.1578495. [DOI] [Google Scholar]
- 64.Chohan ZH, Pervez H, Rauf A, Khan KM, Supuran CT. Antibacterial cobalt (II), copper (II), nickel (II) and zinc (II) complexes of mercaptothiadiazole—Derived furanyl, thienyl, pyrrolyl, salicylyl and pyridinyl Schiff bases. J. Enzyme Inhib. Med. Chem. 2006;21:193–201. doi: 10.1080/14756360500397505. [DOI] [PubMed] [Google Scholar]
- 65.Gaëlle DSY, Yufanyi DM, Jagan R, Agwara MO, Bradshaw D. Synthesis, characterization and antimicrobial properties of cobalt(II) and cobalt(III) complexes derived from 1,10-phenanthroline with nitrate and azide co-ligands. Cogent Chem. 2016;2:1253201. doi: 10.1080/23312009.2016.1253201. [DOI] [Google Scholar]
- 66.Ashajyothi C, Harish KH, Dubey N, Chandrakanth RK. Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: A nanoscale approach. J. Nanostruct. Chem. 2016;6:329–341. doi: 10.1007/s40097-016-0205-2. [DOI] [Google Scholar]
- 67.Park HJ, Park HY, Cha S, Ahn CH, Roh J, Park S, Kim S, Choi K, Yi J, Kim Y, Yoon J. Removal characteristics of engineered nanoparticles by activated sludge. Chemosphere. 2013;92:524–528. doi: 10.1016/j.chemosphere.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Wang X, Yan Y, Hao B, Chen G. Biomimetic layer-by-layer deposition assisted synthesis of Cu, N co-doped TiO2 nanosheets with enhanced visible light photocatalytic performance. Dalt. Trans. 2014;43:14054–14060. doi: 10.1039/C4DT01605J. [DOI] [PubMed] [Google Scholar]
- 69.Liu W, Sun W, Borthwick AGL, Ni J. Comparison on aggregation and sedimentation of titanium dioxide, titanate nanotubes and titanate nanotubes-TiO2: Influence of pH, ionic strength and natural organic matter. Colloids Surfaces A Physicochem. Eng. Asp. 2013;434:319–328. doi: 10.1016/j.colsurfa.2013.05.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.