Abstract
Objective: This study aims to evaluate outcome after conservative management (no pharmacological/surgical intervention other than fluid restriction, diuretics, or ventilator adjustments) compared with active (pharmacological and/or surgical) treatment for patent ductus arteriosus (PDA) in preterm infants and analyze differences in outcome between randomized controlled trials (RCTs) and cohort studies.
Study Design: This is a systematic literature review using PubMed, EMBASE, and Cochrane library. RCTs and cohort studies comparing conservative management with active treatment were included. Meta-analysis was used to compare conservative management with any active (pharmacological and/or surgical), any pharmacological (non-prophylactic and prophylactic), and/or surgical treatment for mortality as primary and major neonatal morbidity as secondary outcome measure. Fixed-effect analysis was used, unless heterogeneity (I2) was >50%. Outcome is presented as relative risk (RR) with 95% confidence interval.
Results: Twelve cohort studies and four RCTs were included, encompassing 41,804 and 720 patients, respectively. In cohort studies, conservative management for PDA was associated with a significantly higher risk for mortality (RR, 1.34 [1.12–1.62]) but a significantly lower risk for bronchopulmonary dysplasia (RR, 0.55 [0.46–0.65]), necrotizing enterocolitis (RR, 0.85 [0.77–0.93]), intraventricular hemorrhage (RR, 0.88 [0.83–0.95]), and retinopathy of prematurity (RR, 0.47 [0.28–0.79]) compared with any active PDA treatment. Meta-analysis of the RCTs revealed no significant differences in outcome between conservative management and active treatment.
Conclusion: No differences in mortality or morbidity for conservative management compared with active treatment regimens were observed in RCTs. Findings from cohort studies mainly highlight the lack of high-quality evidence for conservative management for PDA in preterm infants.
Keywords: PDA, ibuprofen, indomethacin, paracetamol, ligation, placebo, morbidity, mortality
Introduction
A patent ductus arteriosus (PDA) is very common in very low birth weight (VLBW) infants (<1,500 g) (1). It is associated with mortality and severe morbidity, such as bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), and retinopathy of prematurity (ROP) (2).
Prophylactic treatment with indomethacin has been shown to reduce the incidence of symptomatic PDA, ligation, and severe IVH (3). Network meta-analysis of randomized controlled trials (RCTs) showed a significant effect on ductus arteriosus (DA) closure in pharmacologically treated children compared with placebo/no treatment (4). However, effective closure of the DA has not resulted in an improvement either in survival or major neonatal morbidity. When analyzing these RCTs, one has to be aware of the great heterogeneity in used definitions (and therefore inclusion criteria) for hemodynamically significant PDA (hsPDA) (5) and the remarkably high rate of open-label-treated patients in the control group (6). Ligation is an effective strategy to close the DA as well but has been associated with adverse outcome (7). Recent studies that adjusted for confounders prior to ligation, showed no association between ligation and adverse outcome (8, 9).
In the last decade, there has been a shift from aggressive pharmacological and/or surgical treatment toward a more conservative management (10). This change in policy can be justified by two arguments. First, since pharmacological treatment is not associated with an improvement in overall outcome (3, 4, 6, 11), fragile preterm infants are withheld from possible adverse effects of the pharmacological intervention. Second, there is a substantial rate of spontaneous closure of the DA (12, 13), even after failed pharmacological treatment (14). In summary, it is perceived that closure of the DA is delayed in preterm infants and treatment seems to only accelerate closure without improving outcome.
In this review and meta-analyses, we systematically reviewed the literature regarding mortality and morbidity associated with a conservative management for PDA in preterm and/or VLBW infants and compare most relevant outcome measures between RCTs and cohort studies.
Methods
We performed a systematic literature review on 1 July 2020 in PubMed, EMBASE, and Cochrane library using the following search terms “preterm infant,” “very low birth weight infant,” “PDA,” “conservative treatment,” and “placebo” (Table 1). We excluded articles before 2000, as in this period antenatal corticosteroids and surfactant were not part of routine care. Reference lists of reviews and included articles were screened for additional studies. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was followed (15). No review protocol was published.
Table 1.
Search strategy.
| ID | Search | Hits |
|---|---|---|
| PubMed | ||
| #1 | Ductus arteriosus, patent [MeSH Terms] | 8,951 |
| #2 | Patent ductus arteriosus [Title/Abstract] | 8,328 |
| #3 | Patency of the ductus arteriosus [Title/Abstract] | 198 |
| #4 | Persistent ductus arteriosus [Title/Abstract] | 490 |
| #5 | hsPDA [Title/Abstract] | 190 |
| #6 | PDA [Title/Abstract] | 12,050 |
| #7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 21,577 |
| #8 | Infant, extremely premature [MeSH Terms] | 2,477 |
| #9 | Preterm [Title/Abstract] | 75,235 |
| #10 | VLBW [Title/Abstract] | 33,696 |
| #11 | Very low birth weight infant [Title/Abstract] | 485 |
| #12 | Extremely premature infant [Title/Abstract] | 145 |
| #13 | Premature birth [Title/Abstract] | 3,607 |
| #14 | Prematurity [Title/Abstract] | 21,967 |
| #15 | Infant, low birth weight [MeSH Terms] | 34,180 |
| #16 | #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 | 114,537 |
| #17 | Conservative treatment [MeSH Terms] | 2,955 |
| #18 | Conservative [Title/Abstract] | 105,930 |
| #19 | Expectative [Title/Abstract] | 141 |
| #20 | Expectant [Title/Abstract] | 5,866 |
| #21 | Placebo [Title/Abstract] | 214,408 |
| #22 | Placebos [MeSH Terms] | 34,946 |
| #23 | No treatment [Title/Abstract] | 30,200 |
| #24 | #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 | 365,730 |
| #25 | #7 AND #16 AND #24 | 214 |
| #26 | #25 with filters: Publication date from 01 Jan 2000 | 175 |
| EMBASE | ||
| #1 | Patent ductus arteriosus.ti. or patent ductus arteriosus.ab. | 9,985 |
| #2 | PDA.ti. or PDA.ab. | 16,176 |
| #3 | Patency of the ductus arteriosus.ti. or patency of the ductus arteriosus.ab. | 276 |
| #4 | Persistent ductus arteriosus.ti. or persistent ductus arteriosus.ab. | 558 |
| #5 | hsPDA.ti. or hsPDA.ab. | 162 |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 | 22,852 |
| #7 | Extremely preterm infant.ti. or extremely preterm infant.ab. | 161 |
| #8 | Extremely premature infant.ti. or extremely premature infant.ab. | 156 |
| #9 | Extreme preterm infant.ti. or extreme preterm infant.ab. | 15 |
| #10 | Extreme premature infant.ti. or extreme premature infant.ab. | 17 |
| #11 | Very low birth weight.ti. or very low birth weight.ab. | 9,275 |
| #12 | VLBW.ti. or VLBW.ab. | 5,016 |
| #13 | Prematurity.ti. or prematurity.ab. | 28,702 |
| #14 | Premature birth.ti. or premature birth.ab. | 4,534 |
| #15 | #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 | 42,047 |
| #16 | Conservative treatment.ti. or conservative treatment.ab. | 39,060 |
| #17 | Conservative.ti. or conservative.ab. | 137,261 |
| #18 | Expectative.ti. or expectative.ab. | 214 |
| #19 | No treatment.ti. or no treatment.ab. | 44,953 |
| #20 | Placebo.ti. or placebo.ab. | 307,386 |
| #21 | Expectant.ti. or expectant.ab. | 7,805 |
| #22 | #16 OR #17 OR #18 OR #19 OR #20 OR #21 | 489,991 |
| #23 | #6 AND #15 AND #22 | 117 |
| #24 | Limit #23 to year = “2000–current” | 93 |
| Cochrane library | ||
| #1 | MeSH descriptor: [Ductus Arteriosus, Patent] explode all trees | 285 |
| #2 | (Patent ductus arteriosus):ti,ab | 750 |
| #3 | (PDA):ti,ab | 959 |
| #4 | (Persistent ductus arteriosus):ti,ab | 92 |
| #5 | (hsPDA):ti,ab | 33 |
| #6 | (Patency of the ductus arteriosus):ti,ab | 34 |
| #7 | (#6 OR #5 OR #4 OR #3 OR #2 OR #1) | 1,403 |
| #8 | (Extremely preterm):ti,ab | 564 |
| #9 | MeSH descriptor: [Infant, Extremely Premature] explode all trees | 177 |
| #10 | MeSH descriptor: [Infant, Low Birth Weight] explode all trees | 2,157 |
| #11 | (Preterm):ti,ab | 13,188 |
| #12 | (VLBW):ti,ab | 896 |
| #13 | (Very low birth weight infants):ti,ab | 1,680 |
| #14 | (Infant, Extremely Premature):ti,ab | 103 |
| #15 | (Premature birth):ti,ab | 2,672 |
| #16 | (Prematurity):ti,ab | 2,349 |
| #17 | #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 | 17,085 |
| #18 | MeSH descriptor: [Conservative Treatment] explode all trees | 119 |
| #19 | (Conservative):ti,ab | 8,311 |
| #20 | (Expectative):ti,ab | 90 |
| #21 | (Expectant):ti,ab | 1,097 |
| #22 | (Placebo):ti,ab | 293,798 |
| #23 | (No treatment):ti,ab | 262,232 |
| #24 | MeSH descriptor: [Placebos] explode all trees | 23,914 |
| #25 | #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 | 489,460 |
| #26 | #7 AND #17 AND #25 | 339 |
| #27 | #26 with Cochrane Library publication date between Jan 2000 and 1 Jul 2020 | 282 |
Articles were included when it concerned (a) preterm infants <32 weeks' gestation or VLBW infants <1,500 g, with a PDA (irrespective of diagnostic criteria) in (b) a RCT or cohort study with (c) at least one study group managed conservatively (defined as <25% open-label pharmacological treatment with ibuprofen, indomethacin, or paracetamol and/or ligation/endovascular closure for RCTs and <25% active treatment during follow-up for cohort studies) as our aim is to compare active treatment with conservative management instead of “delayed” treatment and when (d) data about the primary outcome (mortality) or secondary outcome measures (BPD, NEC, IVH, and ROP) were available. Conservative management was defined as the absence of any pharmacological or surgical/endovascular intervention with the intention to actively close the DA other than fluid restriction, diuretics, and/or ventilator adjustments.
Articles were excluded if: (a) data about outcome measurements were not available per treatment regimen; (b) language was not English, German or Dutch; and (c) the paper was a conference abstract.
Two reviewers (TH and EJ) screened the title and abstract of the retrieved papers. Disagreements were resolved by a third reviewer (WdB). For eligible studies, corresponding authors were contacted for missing data from subgroups. Two reviewers (TH and EJ) assessed the risk of bias with the Cochrane Risk of Bias Tool for included RCTs (16) and the Newcastle-Ottawa Scale (NOS) for cohort studies (17). Disagreements were resolved by a third reviewer (WdB).
Data extraction regarding study design, study population, definition of (hs)PDA (not specified, clinical parameters only, echocardiographic parameters only, or both clinical and echocardiographic parameters), definition of conservative management (respiratory adjustments, fluid restriction and/or diuretics, or no pharmacological/surgical PDA treatment), percentage open-label active treatment (pharmacological and/or surgical) in the conservative management group, and outcome parameters (mortality, BPD, NEC, IVH, and ROP) from included studies were done by two reviewers (TH and EJ).
If available from the cohort studies, adjusted odds ratio (aOR) was also extracted and expressed as conservative management group compared with the active treatment regimen.
Conservative management was compared with five active treatment regimens, namely (1) any active treatment, defined as treatment with either ibuprofen, indomethacin, or paracetamol and/or ligation/endovascular closure; (2) any pharmacological treatment, defined as treatment with ibuprofen, indomethacin, and/or paracetamol, both prophylactic and non-prophylactic; (3) non-prophylactic pharmacological treatment, defined as treatment with ibuprofen, indomethacin, and/or paracetamol beyond a postnatal age of 24 h; (4) prophylactic pharmacological treatment, defined as treatment with ibuprofen, indomethacin, or paracetamol within a postnatal age of 24 h irrespective of PDA status; and (5) ligation/endovascular closure, defined as ligation/endovascular closure without preceding pharmacological treatment.
Some studies included a subgroup without a PDA. Those subgroups were excluded from the initial analysis, but in a subgroup analysis, we included those low-risk patients in the conservative treatment group to investigate their modulating effect on outcome measures. Furthermore, our main inclusion criterium for PDA was irrespective of diagnostic criteria used. In a subgroup analysis, we will only include studies with an echocardiographically confirmed PDA >1.5 mm in both subgroups.
Outcome measures were entered in Review Manager Software for meta-analysis (Revman version 5.3 Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014). Meta-analysis was performed for RCTs and cohort studies separately per defined treatment regimen. We used random effect if the heterogeneity (I2) was >50% (18) and fixed effect in case of a lower heterogeneity. Effects are presented as risk ratio (RR) and risk difference (RD) with 95% confidence interval (95% CI).
The methodological quality of the studies' outcome parameters was examined with the GRADE method (19). We assessed imprecision as serious if the total number of events was <300 or if the width of the CI of the RR was >0.25. We used the GRADE-pro GDT 2016 software (GRADEpro Guideline Development Tool [Software], McMaster University, 2015) to create a “summary of findings” table to report the quality of evidence. The GRADE approach results in an assessment of the evidence in one of four grades of evidence: high, moderate, low, or very low certainty.
Results
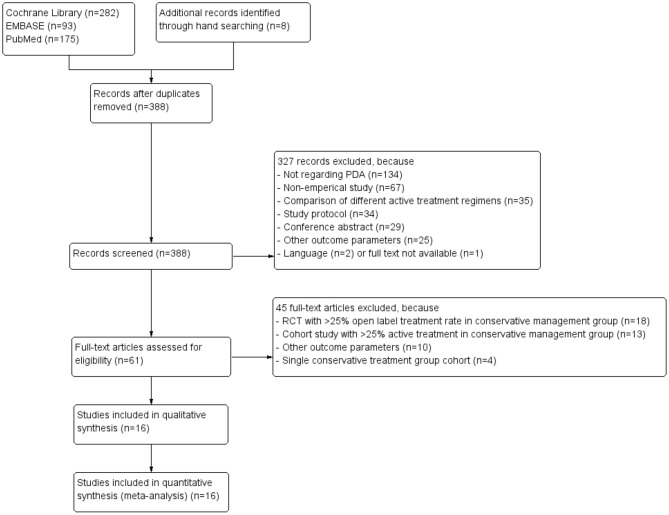
Our search revealed 388 unique articles, of which four RCTs (20–23) and 12 cohort studies (7, 24–34) could be included in the meta-analyses. Figure 1 depicts the PRISMA flow diagram showing the retrieval process of the included articles (15). Due to our inclusion criterium of strict conservative management, we had to exclude many RCTs (n = 18) because of >25% open-label active treatment and cohort studies (n = 13) because of >25% active treatment during follow-up in the conservative management arm.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram of systematic literature review (15).
Study Characteristics
A total of 63,254 patients were analyzed, of which 720 patients from RCTs and 41,804 from cohort studies were included in the initial meta-analyses. The remaining (n = 20,730) were classified as a subgroup without a PDA in four cohort studies and did not receive any (prophylactic) treatment (26, 29) and were therefore excluded from the initial analyses.
In Table 2, the characteristics of the included RCTs and cohort studies are shown. The used definitions for a (hs)PDA varied extensively between studies. Patient characteristics are presented in Table 3.
Table 2.
Study characteristics of included studies.
| References | Period | Design | Treatment strategies | Main inclusion criteria | Main exclusion criteria | Infants (n) | Conservative management | Used drug(s) (dosage if available) | (hs)PDA diagnosis | hsPDA definition | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RCTs | Härkin et al. (20) | 2013 2015 |
Double-blind Monocenter |
CONS (placebo) vs. PPT | GA <32 weeks | CM, lethal disease, PPHN | 48 | Placebo (0.45% saline) | Paracetamol (once 20 mg/kg; 24 × 7.5 mg/kg every 6 h) | CLIN + ECHO | CLIN: increased respiratory support, decreased blood pressure, increased pulse pressure, pulmonary congestion, cardiomegaly, hepatomegaly, murmur, hyperdynamic precordium, bounding pulses ECHO: LA/Ao >1.4, PDA diameter/LPA >1.5, large LtR shunt |
| Kumar Nair et al. (21) | 1998 2001 |
Non-blinded Monocentre |
CONS vs. PPT | BW 750–1,250 g, absence of IVH prior to inclusion | GA <26 weeks, AS5 <5, CM, sepsis, contraindication for PPT | 115 | Not defined | INDO (3 × 0.1 mg/kg/day) start 6–12 h PNA | CLIN + ECHO | CLIN: hemorrhagic pulmonary edema, cardiomegaly on chest X-ray or failure of weaning from ventilatory support ECHO: not defined | |
| Sung et al. (22) | 2014 2019 |
Double-blinded Monocenter | CONS (placebo) vs. PT | GA 23–30-weeks with respiratory support and PDA at PNA 6–14 days | CHD, life threatening CM, predominant RtL shunt, IVH ≥3, contraindication for PT | 142 | Placebo (0.9% saline) | IBU p.o. (10–5–5 mg/kg) | ECHO | ECHO: diameter >1.5 mm with predominant LtR shunt | |
| Van Overmeire et al. (23) | 1999 2001 |
Double-blind Multicenter | CONS (placebo) vs. PPT | GA 24–30 weeks within 6 h PNA | Major CM, IVH > grade I, AS5 <5, sepsis, hypotension, contraindication for PPT | 415 | Placebo | IBU (10–5–5 mg/kg) | Not defined | Not defined | |
| Cohort studies |
Alexander et al. (24) | 1996 2005 |
Retrospective (chart review) Monocentre |
CONS vs. PT or LIG or LIG after PT | BW <1,000 g with ECHO of PDA documented | Not defined | 298 | No PT and/or LIG | INDO (3 × 0.2 mg/kg every 12 h) | ECHO | Not defined |
| Bourgoin et al. (25) | 2003 2011 |
Prospective Multicenter | CONS for (non-hs)PDA vs. PT or LIG for hsPDA | Discharged alive | Dead <2 years, CM, lost to follow-up | 857 | No PT and/or LIG | IBU (10–5–5 mg/kg) | ECHO | PDA diameter >1.5 mm; LA/Ao >1.5; pulsatile flow in the DA; retrograde/absent diastolic flow in the cerebral anterior artery or in the descending thoracic aorta. | |
| Härkin et al. (26) | 2005 2013 |
Retrospective (FMBR database) Multicentre |
CONS vs. PT and/or LIG or no PDA | GA <32 weeks | Mortality <7 days PNA, severe CM | 3,668 | No PT and/or LIG | INDO or IBU | CLIN + ECHO | CLIN: murmur, hyperdynamic precordium, bounding pulses, increased need for respiratory support and increased pulse pressure ECHO: not defined | |
| Heuchan et al. (27) | 2005 2009 |
Retrospective Monocentre | CONS vs. LIG or no PDA | Echocardiogram performed at PNA 6–48 h | CHD | 25 | No PT and/or LIG | None (primary LIG) | CLIN + ECHO | CLIN: murmur, hypotension, pulmonary hemorrhage, renal impairment ECHO: color Doppler diameter | |
| Laughon et al. (28) | 1997 2004 |
Retrospective Multicenter | CONS vs. PPT or STG or LIG or no PDA | GA 23–30-weeks | Not defined | 34,602 | No PT and/or LIG | INDO | Not defined | Not defined | |
| Letshwiti et al. (29) | 2004 2011 |
Retrospective Monocenter | CONS vs. PT (subdivided in ETG and STG) or no PDA | BW <1,500 g | Not defined | 371 | PEEP ≥5 cmH2O, FR (130–150 ml/kg/day) | IBU (10–5–5 mg/kg) | ECHO | PDA diameter >2 mm; LA/Ao >1.5; evidence of reduced splanchnic Doppler flow | |
| Lokku et al. (30) | 2006 2012 |
Retrospective (CNN database) Multicentre |
CONS vs. PT and/or LIG | GA 23–32-weeks with CLIN and/or ECHO of PDA | Dead <72 h PNA, severe CM, ≥triplet, missing data regarding date of birth or sex | 5,824 | No PT and/or LIG | INDO | CLIN ± ECHO | CLIN: systolic murmur, bounding pulses, wide pulse pressure ECHO: not defined | |
| Mirea et al. (7) | 2004 2008 |
Retrospective (CNN database) Multicenter |
CONS vs. PT and/or LIG | GA ≤ 32 weeks with CLIN and/or ECHO of PDA | Dead <72 h PNA, CHD | 3,556 | FR and/or diuretics | INDO | CLIN ±- ECHO | Not defined | |
| Mohamed et al. (31) | 2001 2014 |
Retrospective (database) Monocentre |
CONS vs. PT | BW <1,500 g | Not defined | 643 | Standard respiratory setting, no FR | INDO [2001–2006] IBU [2006–2014] | CLIN + ECHO | CLIN: cardiac murmur, pulsating pericardium, wide peripheral pulses, | |
| 2014 | increasing metabolic acidosis (base excess < -8 mEq/L) ECHO: moderate to severe PDA | ||||||||||
| Sadeck et al. (32) | 2010 2011 |
Retrospective (BNRN database) Multicentre |
CONS vs. PT and/or LIG | BW <1,000 g, GA <33 weeks with ECHO of PDA | Died or transferred <3 days PNA, congenital infections, CM | 494 | No PT and/or LIG | INDO/IBU | Not defined | Not defined | |
| Slaughter et al. (33) | 2006 2013 |
Retrospective (PHIS database) Multicentre |
CONS vs. PT | GA <28 weeks | Hospitalized <3 days, admitted >24 h PNA, no recorded discharge status | 12,018 | No PT and/or LIG | INDO/IBU | Not defined | Not defined | |
| Sung et al. (34) | 2009 2014 |
Retrospective (database) Monocentre |
CONS vs. PT and/or LIG | GA 23–26- weeks | Died <48 h, CHD, PDA <2 mm or off ventilator | 178 | FR and diuretics if indicated | INDO (3 × 0.2 mg/kg every 12 h) | CLIN + ECHO | CLIN: deterioration in respiratory condition, cardiac murmur, hyperactive precordium, | |
| hypotension and widened pulse pressure ECHO: PDA diameter ≥2 mm with predominant LtR shunt |
AS5, Apgar score at 5 min postpartum; BNRN, Brazilian Neonatal Research Network; BW, birth weight; CHD, congenital heart disease; CM, congenital malformation and/or chromosomal abnormality and/or genetic syndrome; CNN, Canadian Neonatal Network; CLIN, clinical diagnosis; CONS, conservative management; d, days; DA, ductus arteriosus; ECHO, echocardiographic parameters; ETG, early treatment group; FMBR, Finnish Medical Birth Register; FR, fluid restriction; GA, gestational age; IBU, ibuprofen; INDO, indomethacin; IVH, intraventricular hemorrhage; LA/Ao, left atrium to aortic root ratio; LIG, ligation; LPA, left pulmonary artery; LtR, left to right; (hs)PDA, (hemodynamically significant) patent ductus arteriosus; PEEP, positive end expiratory pressure; PHIS, Pediatric Health Information System; PNA, postnatal age; PPHN, persistent pulmonary hypertension of a newborn; PT, pharmacological treatment; PPT, prophylactic pharmacological treatment; RCTs, randomized controlled trials; RtL, right to left; STG, standard treatment group.
Table 3.
Patient characteristics of included studies.
| Patient characteristics | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conservative management | Prophylactic pharmacological treatment |
Pharmacological treatment | Ligation closure | Pharmacotherapy and/or ligation closure |
|||||||||||||
| References | Infants (male/N) |
GA (weeks) | BW (grams) | Open-label treatment |
Infants (male/N) |
GA (weeks) | BW (g) | Infants (male/N) |
GA (weeks) | BW (g) | Infants (male/N) |
GA (weeks) | BW (g) | Infants (male/N) |
GA (weeks) | BW (g) | |
| RCTs | Härkin et al. (20) | 14/25 | 28.3 ± 2.06 | 1,120 ± 340 | 12.0% | 13/23 | 28.4 ± 2.36 | 1,220 ± 430 | – | – | – | – | – | – | – | – | – |
| Kumar Nair et al. (21) | NA/59 | 27.9 ± 1.4 | 995 ± 83.6 | NA | NA/56 | 27.8 ± 1.2 | 989.5 ± 93.5 | – | – | – | – | – | – | – | – | – | |
| Sung et al. (22) | 41/72 | 26.7 ± 2.0 | 915 ± 243 | 0% | – | – | – | 28/70 | 26.8 ± 2.1 | 893 ± 256 | – | – | – | – | – | – | |
| Van Overmeire et al. (23) | NA/210 | 28.1 ± 1.6 | 1,065 ± 324 | 24.8% | NA/205 | 28.1 ± 1.7 | 1,048 ± 315 | – | – | – | – | – | – | – | – | – | |
| Cohort studies |
Alexander et al. (24) | NA/54 | 25.7 ± 1.9 | 729.6 ± 169.6 | 0% | – | – | – | ?/198 | 26.1 ± 1.9 | 739 ± 140.5 | ?/46 | 24.8 ± 1.5 | 678.7 ± 153.5 | – | – | – |
| Bourgoin et al. (25) | 254/505 | NA | 977 ± 212 | 0% | – | – | – | 134/248 | NA | 911 ± 191 | 40/104 | NA | 833 ± 225 | – | – | – | |
| Härkin et al. (26) | 98/182 | 28.82 ± 2.41 | 1,225 ± 402 | 0% | – | – | – | 395/770 | 26.3 ± 1.2 | 1,115 ± 336 | 66/112 | 25.6 ± 1.4 | 834 ± 297 | 134/250 | 25.5 ± 1.3 | 846 ± 278 | |
| Heuchan et al. (27) | 4/7 | 27 [25–28] | 1,046 [680–1,440] | 0% | – | – | – | – | – | – | 8/11 | 26 [24–27] | 780 [613–1,240] | – | – | – | |
| Laughon et al. (28) | 2,201/ 3,886 |
27 [26–29] | 970 [750–1,220] | 0% | 3,293/ 6,189 |
26 [25–28] | 873 [703–1075] | 3,021/ 5,690 |
27 [25–29] | 960 [760–1,205] | 388/701 | 25 [24–27] | 730 [624–895] | – | – | – | |
| Letshwiti et al. (29)§ | 16/34 | 27.4 ± 2.7 | 1,010 ± 250 | 14.7% | – | – | – | 15/52 | 27.9 ± 2.0 | 1,040 ± 270 | – | – | – | – | – | – | |
| 26/52 | 27.5 ± 1.9 | 1,010 ± 280 | |||||||||||||||
| Lokku et al. (30)† | 811/ 1,486 |
28.2 ± 2.3 | NA | 0% | – | – | – | 1,754/ 3,226 |
27.1 ± 2.1 | NA | 165/280 | 26.0 ± 2.2 | NA | 423/832 | 25.6 ± 1.7 | NA | |
| 28.2 ± 2.4 | NA | 26.6 ± 2.0 | NA | 26.0 ± 1.8 | NA | 25.3 ± 1.6 | NA | ||||||||||
| Mirea et al. (7) | 321/577 | 28.3 ± 2.3 | NA | 0% | – | – | – | 1,062/ 2,026 |
27.0 ± 2.1 | NA | 185/327 | 26.0 ± 2.3 | NA | 325/626 | 25.5 ± 1.7 | NA | |
| Mohamed et al. (31) | 122/228 | 28.0 ± 3.4 | 1,016 ± 340 | 0% | – | – | – | 216/415 | 27.7 ± 2.9 | 988 ± 311 | – | – | – | – | – | – | |
| Sadeck et al. (32) | 91/187 | 27.6 ± 2.2 | 772 ± 142.3 | 0% | – | – | – | 90/205 | 27.4 ± 1.9 | 804 ± 121.6 | 48/102 | 26.6 ± 1.8 | 781 ± 118.5 | – | – | – | |
| Slaughter et al. (33) | 4,302/ 8,130 |
NA | NA | 0% | – | – | – | 2,068/ 3,888 |
NA | NA | – | – | – | – | – | – | |
| Sung et al. (34) | 54/97 | 24.5 ± 1.0 | 718 ± 137 | 0% | – | – | – | – | – | – | 35/81 | 24.6 ± 1.1 | 728 ± 134 | – | – | – | |
Data presented as number, percentage, mean ± standard deviation or median [interquartile range]. Statistical difference between other groups in same study are bold.
ETG; early treatment group, GA; gestational age, N; total infants, NA; not available, PDA; patent ductus arteriosus, RCTs; randomized controlled trials, STG; symptomatic treatment group.
Pharmacological treatment in study subdivided between early treatment and symptomatic treatment.
Cohort subdivided in cohort 2006–2008 and 2009–2012. BW; birth weight.
Three RCTs were placebo controlled (20, 22, 23), while for one RCT, the control arm was not specified (21). In the cohort studies, the definition of conservative management ranged from no treatment at all to a regimen with fluid restriction, diuretics, and/or adaptation in ventilator settings or the absence of any pharmacological/surgical treatment.
Mortality was heterogeneously defined, since four studies excluded early neonatal deaths within 24–72 postnatal hours (7, 30, 32, 34). The outcome parameter BPD was defined according to the international criteria at 36 weeks postmenstrual age in 12 papers (7, 20, 22, 26–34). NEC was defined according to the Bell stadium in nine studies (7, 20, 22, 23, 26, 27, 30, 31, 34). Thirteen studies defined IVH as grade III or higher (7, 20–23, 25–32, 34), ROP was defined as stage 3 or higher in five studies (7, 22, 26, 28, 34). No study described endovascular closure.
Risk of Bias
The quality of the included RCTs was high, given the low risk of bias (Table 4). The quality of the cohort studies was classified as moderate (Table 5).
Table 4.
Risk of bias assessment of included randomized controlled trials according to the cochrane risk of bias tool (16).
Table 5.
Risk of bias assessment of included cohort studies according to Newcastle–Ottawa scale (NOS) (17).
| References | Selection | Comparability | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Alexander et al. (24) | * | * | * | * | ** | * | – | * |
| Bourgoin et al. (25) | * | * | * | * | ** | * | * | * |
| Härkin et al. (26) | * | * | * | * | ** | * | * | * |
| Heuchan et al. (27) | * | * | * | * | – | * | – | * |
| Laughon et al. (28) | – | * | * | * | ** | * | – | * |
| Letshwiti et al. (29) | * | * | * | * | – | * | – | * |
| Lokku et al. (30) | * | * | * | * | * | * | – | * |
| Mirea et al. (7) | * | * | * | * | ** | * | * | * |
| Mohamed et al. (31) | * | * | * | * | ** | * | * | * |
| Sadeck et al. (32) | * | * | * | * | – | * | – | * |
| Slaughter et al. (33) | * | * | * | * | * | * | * | * |
| Sung et al. (34) | * | * | * | * | ** | * | – | * |
A; representatives of the exposed cohort, B; selection of non-exposed cohort, C; ascertainment of exposure, D; demonstration that outcome of interest was not present at start of study, E; comparability of cohorts on the basis of the design or analysis, F; assessment of outcome, G; was follow-up long enough for outcomes to occur, H; adequacy of follow-up of cohorts.
Maximum score for each item is one star (*), except for comparability for which two stars (**) can be scored, and if not scored positive presented as “–”.
Meta-Analysis
Meta-Analysis Outcome Measures Randomized Controlled Trials
Meta-analysis of the four included RCTs did not show significant differences for mortality or morbidity in any of the predefined groups, as is shown in Table 6 (Supplementary Material 1). The quality of the evidence was graded as moderate to low (Supplementary Material 2).
Table 6.
Outcome measurements after meta-analysis.
| Comparison | (Sub)group | Mortality | BPD (any definition) | NEC (any stage) | IVH (any grade) | ROP (any stage) |
|---|---|---|---|---|---|---|
| Conservative management vs. any active treatment | RCTs | 1.09§ (0.73–1.61) | 0.80 (0.55–1.17) | 1.17§ (0.65–2.13) | 1.00§ (0.71–1.40) | 0.85§ (0.45–1.60) |
| 0.01 (−0.04 to 0.06) | −0.06 (−0.16 to 0.04) | 0.01 (−0.02 to 0.04) | 0.00 (−0.04 to 0.05) | −0.03 (−0.13 to 0.08) | ||
| [4; 720] | [4; 709] | [4; 720] | [4; 720] | [2; 190] | ||
| Cohort | 1.34 (1.12–1.62) | 0.55 (0.46–0.65) | 0.85§ (0.77–0.93) | 0.88§ (0.83–0.95) | 0.47 (0.28–0.79) | |
| studies | 0.03 (0.01–0.06) | −0.18 (−0.24 to −0.12) | −0.01 (−0.02 to −0.01) | −0.02 (−0.03 to −0.01) | −0.06 (0.10 to −0.02) | |
| [11; 40,916] | [11; 39,993] | [9; 28,004] | [10; 28,504] | [8; 26,608] | ||
| Conservative management vs. any pharmacological treatment | RCTs | 1.09§ (0.73–1.61) | 0.80 (0.55–1.17) | 1.17§ (0.65–2.13) | 1.00§ (0.71–1.40) | 0.85§ (0.45–1.60) |
| 0.01 (−0.04 to 0.06) | −0.06 (−0.16 to 0.04) | 0.01 (−0.02 to 0.04) | 0.00 (−0.04 to 0.05) | −0.03 (−0.13 to 0.08) | ||
| [4; 720] | [4; 709] | [4; 720] | [4; 720] | [2; 190] | ||
| Cohort | 1.46 (1.14–1.85) | 0.63 (0.51–0.78) | 1.06 (0.78–1.46) | 0.95§ (0.89–1.02) | 0.57 (0.40–0.82) | |
| studies | 0.05 (0.01–0.08) | −0.12 (−0.17 to −0.06) | 0.01 (−0.02 to 0.03) | −0.01 (−0.02 to 0.00) | −0.04 (−0.06 to −0.02) | |
| [7; 24,729] | [7; 24,104] | [6; 23,965] | [7; 24,446] | [5; 22,892] | ||
| Conservative management vs. nonprophylactic pharmacological treatment | RCTs | 0.97§ (0.33–2.87) | 0.89§ (0.60–1.32) | 0.42§ (0.11–1.55) | 1.94§ (0.37–10.28) | 0.91§ (0.47–1.74) |
| 0.00 (−0.09 to 0.09) | −0.05 (−0.22 to 0.12) | −0.06 (−0.14 to 0.03) | 0.03 (−0.04 to 0.09) | −0.02 (−0.15 to 0.11) | ||
| [1; 142] | [1; 131] | [1; 142] | [1; 142] | [1; 142] | ||
| Cohort | 1.54 (1.13–2.09) | 0.60 (0.48–0.76) | 1.01 (0.81–1.26) | 0.97§ (0.90–1.04) | 0.57 (0.37–0.87) | |
| studies | 0.04 (0.00–0.07) | −0.13 (−0.18 to −0.07) | 0.00 (−0.02 to 0.01) | 0.00 (−0.02 to 0.01) | −0.04 (−0.06 to −0.01) | |
| [6; 18,148] | [6; 17,779] | [5; 17,640] | [6; 18,141] | [4; 16,567] | ||
| Conservative management vs. prophylactic pharmacological treatment | RCTs | 1.11§ (0.72–1.69) | 0.66 (0.25–1.76) | 1.63§ (0.81–3.31) | 0.96§ (0.68–1.35) | 0.31§ (0.01–7.20) |
| 0.01 (−0.04 to 0.07) | −0.06 (−0.19 to 0.07) | 0.03 (−0.01 to 0.07) | −0.01 (−0.06 to 0.05) | −0.04 (−0.15 to 0.07) | ||
| [3; 578] | [3; 578] | [3; 578] | [3; 578] | [1; 48] | ||
| Cohort | 0.92§ (0.83–1.01) | 0.82§ (0.78–0.87) | 0.77§ (0.67–0.88) | 0.91§ (0.81–1.01) | 0.66§ (0.59–0.75) | |
| studies | −0.01 (−0.03 to 0.00) | −0.07 (−0.09 to −0.05) | −0.02 (−0.03 to −0.01) | −0.01 (−0.03 to 0.00) | −0.05 (−0.06 to −0.03) | |
| [1; 10,075] | [1; 9,580] | [1; 9,580] | [1; 9,580] | [1; 9,580] | ||
| Conservative management vs. ligation | RCTs | NA | NA | NA | NA | NA |
| Cohort | 1.25 (0.76–2.05) | 0.36 (0.27–0.47) | 0.49 (0.35–0.68) | 0.65 (0.48–0.88) | 0.23 (0.18–0.31) | |
| studies | 0.07 (−0.02 to 0.16) | −0.36 (−0.47 to −0.26) | −0.07 (−0.12 to −0.02) | −0.09 (−0.15 to −0.03) | −0.17 (−0.28 to −0.07) | |
| [7; 7,867] | [7; 8,021] | [6; 7,535] | [7; 8,020] | [6; 7,146] |
Data presented as risk ratio (95% confidence interval) and risk difference (95% confidence interval) after random effect unless otherwise specified, [number of studies; number of patients].
BPD; bronchopulmonary dysplasia, IVH; intraventricular hemorrhage, NA; not available, NEC; necrotizing enterocolitis, RCTs; randomized controlled trials, ROP; retinopathy of prematurity.
Fixed effect. Significant differences are depicted in bold fonts. To show difference between risk ratio with 95% confidence interval in non-italic font, risk difference with 95% confidence interval in italic and number of studies/patients within brackets.
Meta-Analysis Outcome Measures Cohort Studies
Meta-analysis of the cohort studies revealed that conservative management was associated with a higher risk for mortality compared with any active treatment (RR, 1.34 [1.12–1.62]; RD, 0.03 [0.01–0.06]), any pharmacological treatment (RR, 1.46 [1.14–1.85]; RD, 0.05 [0.01–0.08]), and non-prophylactic pharmacological treatment (RR, 1.54 [1.13–2.09]; RD, 0.04 [0.00–0.07]) (Table 6; Supplementary Material 3). The quality of the evidence was graded as very low (Supplementary Material 4).
Conservative management was associated with a lower risk for BPD and ROP compared with both each separate active treatment regimen and any active treatment. The risk for NEC was significantly lower for conservative management in comparison with any active treatment (RR, 0.85 [0.77–0.93]; RD, −0.01 [−0.02 to −0.01]), prophylactic pharmacological treatment (RR, 0.77 [0.67–0.88]; RD, −0.02 [−0.03 to −0.01]), and ligation (RR, 0.49 [0.35–0.68]; RD, −0.07 [−0.12 to −0.02]). Conservative management was associated with a lower risk for IVH compared with any active treatment (RR, 0.88 [0.83–0.95]; RD, −0.02 [−0.03 to 0.01]) and ligation (RR, 0.65 [0.48–0.88]; RD, −0.09 [−0.15 to −0.03]) (Table 6; Supplementary Material 3). The quality of the evidence was graded as very low (Supplementary Material 4).
Meta-Analysis Outcome Measures Cohort Studies Including Patients Without a PDA
Subgroup baseline characteristics and outcome measures were available for patients without a PDA in three studies (n = 20,497) (26–28). In this subgroup, analysis outcome of those patients was added to the conservative management group (Table 7). The higher risk for mortality lost significance in almost all subgroups, while the lower risk for morbidity was even more pronounced (Table 8, Supplementary Material 5).
Table 7.
Patient characteristics of cohort studies with subgroup without patent ductus arteriosus.
| Patient characteristics | ||||||
|---|---|---|---|---|---|---|
| Conservative management | No or asymptomatic PDA | |||||
| References | Infants (male/N) | GA (weeks) | BW (grams) | Infants (male/N) | GA (weeks) | BW (g) |
| Härkin et al. (26) | 98/182 | 28.82 ± 2.41 | 1,225 ± 402 | 1,398/2,536† | 29.7 ± 1.9 | 1,340 ± 371 |
| Heuchan et al. (27) | 4/7 | 27 [25–28] | 1,046 [680–1,440] | 3/7 | 26 [24–28] | 912 [500–1,440] |
| Laughon et al. (28) | 2,201/3,886 | 27 [26–29] | 970 [750–1,220] | 9,796/18,136 | 29 [27–30] | 1,170 [895–1,400] |
Data presented as number, mean ± standard deviation or median [interquartile range]. Statistical difference between other treatment groups in same study are in bold.
BW; birth weight, GA; gestational age, N; total infants, PDA; patent ductus arteriosus.
Including 182 patients with PDA that were conservatively managed and analyzed separately.
Table 8.
Outcome measurements after meta-analysis of cohort studies including patient without patent ductus arteriosus.
| Comparison | Mortality | BPD (any definition) | NEC (any stage) | IVH (any grade) | ROP (any stage) |
|---|---|---|---|---|---|
| Conservative management vs. any active treatment | 1.14 (0.91–1.43) 0.02 (−0.01 to 0.04) [11; 61,372] |
0.47 (0.38–0.57)
−0.20 (−0.26 to −0.14) [11; 57,400] |
0.78 (0.61–0.99)
−0.02 (−0.04 to 0.00) [9; 45,831] |
0.71 (0.50–0.99)
−0.04 (−0.07 to −0.01) [10; 46,266] |
0.34 (0.27–0.43)
−0.07 (−0.10 to −0.03) [8; 44,371] |
| Conservative management vs. any pharmacological treatment | 1.20 (0.94–1.54) 0.02 (−0.01 to 0.04) [7; 45,178] |
0.51 (0.42–0.61)
−0.15 (−0.20 to −0.10) [7; 41,504] |
0.92 (0.63–1.35) 0.00 (−0.03 to 0.02) [6; 41,785] |
0.77 (0.53–1.12) −0.02 (−0.05 to 0.01) [7; 42,221] |
0.39 (0.33–0.46)
−0.05 (−0.09 to −0.01) [5; 40,648] |
| Conservative management vs. non-prophylactic pharmacological treatment | 1.20 (0.97–1.49) 0.01 (−0.01 to 0.04) [6; 38,597] |
0.47 (0.39–0.57)
−0.16 (−0.22 to −0.10) [6; 35,179] |
0.83 (0.60–1.15) −0.01 (−0.04 to 0.01) [5; 35.460] |
0.72 (0.50–1.05) −0.03 (−0.06 to 0.00) [6; 35,896] |
0.42 (0.33–0.53)
−0.05 (−0.09 to −0.02) [4; 32,138] |
| Conservative management vs. prophylactic pharmacological treatment |
0.74 (0.69–0.79)
−0.04 (−0.05 to −0.03) [1; 28,211] |
0.54 (0.51–0.56)
−0.19 (−0.20 to −0.17) [1; 25,151] |
0.59 (0.53–0.65) −0.04 (−0.05 to −0.03) [1; 25,151] |
0.44 (0.40–0.48)
−0.07 (−0.08 to −0.07) [1; 25,151] |
0.35 (0.32–0.39)
−0.09 (−0.10 to −0.08) [1; 25,151] |
| Conservative management vs. ligation | 0.96 (0.49–1.87) 0.05 (−0.05 to 0.14) [7; 28,323] |
0.30 (0.20–0.43)
−0.39 (−0.49 to −0.28) [7; 25,428] |
0.40 (0.28–0.57)
−0.09 (−0.14 to −0.03) [6; 25,410] |
0.49 (0.25–0.94)
−0.11 (−0.20 to −0.03) [7; 25,782] |
0.16 (0.14–0.18)
−0.18 (−0.30 to −0.06) [6; 24,909] |
Data presented as risk ratio (95% confidence interval) and risk difference (95% confidence interval) after random effect, [number of studies; number of patients]. Significant differences are depicted in bold fonts.
BPD; bronchopulmonary dysplasia, IVH; intraventricular hemorrhage, NEC; necrotizing enterocolitis, RCTs; randomized controlled trials, ROP; retinopathy of prematurity. To show difference between risk ratio with 95% confidence interval in non-italic font, risk difference with 95% confidence interval in italic and number of studies/patients within brackets.
Meta-Analysis Outcome Measures Cohort Studies With Echocardiographic Defined PDA
We performed a subgroup meta-analysis on the two cohort studies that used echocardiographic definitions (n = 316) (29, 34). Outcome measurements, as presented in Table 9, showed a significant lower risk for BPD in the conservative treated group compared with the available subgroups any treatment and any/non-prophylactic pharmacological treatment. Mortality and other morbidity outcomes showed no difference.
Table 9.
Outcome measurements after meta-analysis of cohort studies including echocardiographic defined patent ductus arteriosus.
| Comparison | Mortality | BPD(any definition) | NEC (any stage) | IVH(any grade) | ROP (any stage) |
|---|---|---|---|---|---|
| Conservative management versus any active treatment | 0.79 (0.35–1.78) | 0.43 (0.32 to 0.58) | 0.94 (0.47 to 1.88) | 0.66 (0.36 to 1.22) | 1.17 (0.39 to 3.54) |
| −0.02 (−0.08 to 0.04) | –0.38 (–0.49 to –0.27) | −0.01 (−0.08 to 0.06) | −0.06 (−0.14 to 0.02) | 0.01 (−0.06 to 0.08) | |
| [2; 316] | [2; 287] | [2; 316] | [2; 316] | [1; 178] | |
| Conservative management versus any pharmacological treatment/non -prophylactic pharmacological treatment | 0.61 (0.07 to 5.06) | 0.38 (0.18 to 0.80) | 0.76 (0.17 to 3.43) | 0.66 (0.20 to 2.14) | NA |
| −0.02 (−0.09 to 0.05) | –0.30 (–0.47 to –0.14) | −0.02 (−0.11 to 0.08) | −0.05 (−0.16 to 0.07) | ||
| [1; 138] | [1; 132] | [1; 138] | [1; 138] |
Data presented as risk ratio (95% confidence interval) and risk difference (95% confidence interval) after fixed effect, [number of studies; number of patients]. Significant differences are depicted in bold fonts. BPD, bronchopulmonary dysplasia; IVH, intraventricular haemorrhage; NA, not available; NEC, necrotizing enterocolitis; RCTs, randomised controlled trials; ROP, retinopathy of prematurity.
To show difference between risk ratio with 95% confidence interval in non-italic font, risk difference with 95% confidence interval in italic and number of studies/patients within brackets.
Adjusted Outcome
Adjusted Outcome Measures From Cohort Studies
Eight cohort studies calculated aOR for baseline characteristics between conservative management and either pharmacological therapy, ligation, or pharmacological therapy followed by ligation (7, 26, 28, 30, 31, 33–35). Table 10 shows a statistically significant higher risk for mortality and an overall lower risk for morbidity, especially BPD, in the conservatively managed group.
Table 10.
Adjusted odds ratio for outcome parameters in non-randomized cohort studies.
| Comparison | References | Adjusted for | Mortality | BPD | NEC | IVH | ROP |
|---|---|---|---|---|---|---|---|
| Conservative management vs. pharmacological treatment | Härkin et al. (26) | GA, SGA, RDS, delivery hospital, and ACS | 2.0 (1.1–3.3) | 0.5 (0.3–0.9) | 0.8 (0.6–1.1) | 0.9 (0.6–1.4) | 0.9 (0.4–2.0) |
| Laughon et al. (28) | BW, GA, inborn status, and ACS | 1.7 (1.4–2.0) | 0.7 (0.6–0.8) | NS | NS | 0.7 (0.6–0.8) | |
| Mohamed et al. (31) | GA, BW, sex, and maternal conditions | 0.51 (0.25–0.99) | 0.71 (0.28–1.80) | 0.84 (0.46–1.53) | 1.31 (0.61–2.81) | 1.31 (0.51–3.38) | |
| Conservative management vs. ligation | Härkin et al. (26) | GA, SGA, RDS, delivery hospital, and ACS | 1.0 (0.6–2.0) | 0.3 (0.1–0.5) | 0.5 (0.3–0.8) | 0.2 (0.1–0.4) | 1.7 (0.3–10.0) |
| Sung et al. (34) | GA, BW, SGA, sex, AS5, and ACS | 0.8§ (0.3–2.2) | 0.4§ (0.2–0.8) | – | – | – | |
| Conservative management vs. pharmacological treatment and/or ligation | Härkin et al. (26) | GA, SGA, RDS, delivery hospital, and ACS | 3.3 (1.4–5.0) | 0.2 (0.1–0.3) | 0.7 (0.4–1.1) | 0.8 (0.5–1.3) | 0.7 (0.3–1.7) |
Data presented as adjusted odds ratio (95% confidence interval) after multivariable logistic regression unless otherwise specified. Significant differences are bold.
Propensity score adjusted.
ACS; antenatal corticosteroids, AS5; Apgar score at 5 min postpartum, BPD; bronchopulmonary dysplasia, BW; birth weight, CS; Cesarean section, GA; gestational age, IVH; intraventricular hemorrhage, NEC; necrotizing enterocolitis, NS; not significant, RDS; respiratory distress syndrome, ROP; retinopathy of prematurity, SGA; small for gestational age.
Adjusted Composite Outcome Measures From Cohort Studies
Studies that calculated an adjusted composite outcome, mainly involving mortality and/or BPD, observed lower aOR after conservative management in comparison with pharmacological treatment (30), to ligation alone (7, 30, 34), and pharmacological therapy and/or ligation (7, 30) (Table 11). One study defined composite outcome as survival without death or BPD and found no difference between conservative treatment and pharmacological treatment (aOR, 1.72 [0.92–3.23]) (31).
Table 11.
Adjusted odds ratio for composite outcome in cohort studies.
| Composite outcome according to comparison | |||||
|---|---|---|---|---|---|
| References | Adjusted for | Cohort | Conservative management vs. pharmacological treatment | Conservative management vs. ligation | Conservative management vs. pharmacological treatment and/or ligation |
| Lokku et al. (30)† | GA, SGA, sex, SNAP II score >20, outborn, maternal hypertension, and ACS | 2006–2008 2009–2012 |
1.05 (0.79–1.41) 0.61 (0.51–0.74) |
0.53 (0.31–0.93) 0.24 (0.13–0.43) |
0.36 (0.23–0.56) 0.27 (0.19–0.38) |
| Mirea et al. (7)† | GA, SGA, sex, SNAP II >20, year of birth, site, inborn/outborn, | 0.91 (0.72–1.16) | 0.56 (0.37–0.87) | 0.43 (0.30–0.60) | |
| maternal hypertension, gestational | 0.99§ (0.76–1.28) | 0.52§ (0.29–0.93) | 0.45§ (0.29–0.70) | ||
| diabetes, chorioamnionitis, and ACS | 0.99§§ (0.72–1.35) | 0.59§§ (0.42–0.82) | 0.80§§ (0.66–0.97) | ||
| Sung et al. (34)†† | GA, BW, SGA, AS5, sex, and ACS | – | 0.5§ (0.2–0.9) | – | |
Data presented as adjusted odds ratio (95% confidence interval) after multivariable logistic regression unless otherwise specified. Significant differences are bold.
ACS; antenatal corticosteroids, AS5; Apgar score at 5 min postpartum, BW; birth weight, GA; gestational age, SGA; small for gestational age, SNAP II; Score for Neonatal Acute Physiology, version II.
Propensity score adjusted.
Propensity score matched.
Composite outcome defined as mortality or any severe morbidity (IVH grade ≥3, or periventricular leukomalacia, ROP stage ≥3, BPD defined as oxygen need at 36 weeks postmenstrual age and NEC stages ≥2).
Composite outcome defined as mortality or BPD.
Discussion
In this systematic review, we reviewed the available literature of the last two decades regarding a conservative management for a PDA in preterm infants. Meta-analysis of the included RCTs showed no differences in outcome for the conservative management group compared with active treatment groups. This is in line with a recent network meta-analysis that demonstrated no differences in severe neonatal morbidities between pharmacological treatment and no (active) treatment (4). This meta-analysis also included RCTs with an overall high rate of open-label active treatment in the conservative management (no treatment/placebo) group. However, our meta-analysis only including strict conservative management regimens RCTs, also showed no differences in mortality and/or morbidity in the small number of patients included.
Contrarily, meta-analysis of the cohort studies suggest an association with a significant higher risk for mortality in the conservative management group compared with most active treatment groups. Our meta-analysis hereby adds to the available evidence indicating an association between PDA and mortality (2); however, causality remains unproven. Remarkably, a significant lower risk for severe neonatal morbidities was found in our meta-analysis of the cohort studies in the conservative management group compared with various active treatment regimens.
The risk of bias of the included cohort studies was classified as moderate. The main risk of bias was treatment selection bias or confounding by indication, since patients could be managed conservatively due to contraindications for ibuprofen/indomethacin or because of a non-hsPDA. The lower incidence of neonatal morbidity might be due to survival bias, as patients who died cannot develop BPD. Furthermore, patients at the highest risk to develop (severe) morbidities are more likely to die. This might be enhanced by the exclusion of early neonatal death in four cohort studies (7, 30, 32, 34). Many cohort studies were derived from databases (7, 26, 30–34), which are at risk of poor diagnostic precision. These biases might explain the observed higher risk for mortality on the one hand, and the lower risk for morbidity on the other hand for conservatively managed patients compared with active treatment regimens.
Our subgroup analysis including patients without a PDA (“best-case scenario”) further suggests treatment selection bias, as patients with the highest a priori risk for mortality were possibly not treated for their PDA while low-risk patients might have been excluded from retrospective cohort studies. The higher risk for mortality lost significance, while the lower risk for morbidity was even more pronounced. This supports our hypothesis that the decreased risk for morbidity might be due to the inclusion of relatively well children in the conservative treatment group. Furthermore, treated patients were systematically younger and/or smaller than conservatively treated patients. We also included a subgroup analysis of patients with an echocardiographic confirmed PDA (“worst-case scenario”) in an attempt to exclude preterm infants with a small PDA that did not necessitated treatment as it would close spontaneously. In this subgroup, only the risk for BPD was significantly lower for the conservative management group. This might be due to the clinicians' tendency to treat a PDA, even with the same echocardiographic PDA characteristics, in case of ventilator dependency which in itself is a risk factor for BPD (36).
The higher risk for mortality and lower risk for morbidity in conservatively managed infants remained significant in three cohort studies after adjustment for baseline characteristics (26, 28, 34). Only one study observed a significantly lower risk for mortality for conservatively managed infants, without a difference in risk for morbidity. This might be due to differences in neonatal practice overall, since they compared a first epoch characterized by active pharmacological treatment (2001–2009) with a second epoch with predominantly a conservative management (2010–2014) (31). The composite outcome, heterogeneously defined as mortality and/or morbidity, was significantly lower in the conservatively managed group (7, 30, 34).
Adjustment for baseline perinatal characteristics does not completely reduce treatment selection bias in the cohort studies, since they cannot correct for all relevant clinical conditions after birth and potential unmeasured confounders. These confounders might have influenced the clinician's decision whether or not to treat a PDA in an infant. The importance of these confounders might be crucial, since in analogy the association between ligation and morbidity lost significance only after the adjustment for postnatal, preligation covariates like sepsis, cardiovascular drug support, NEC, and severe IVH (9).
We could not replicate the finding that prophylactic treatment significantly reduces the risk of IVH (3). This might be due to our exclusion criteria, since most trials regarding prophylactic indomethacin were conducted before 2000 and/or had >25% open-label active treatment in the placebo group (37). In the only included cohort comparing conservative management to prophylactic treatment, although conservatively treated infants were significantly less mature, there was no difference in IVH in both the adjusted and unadjusted analysis (28).
This systematic literature review highlights the main pitfalls of the available evidence regarding PDA treatment in preterm and/or VLBW infants. Eligible RCTs are scarce, due to our strict inclusion criteria. Consequentially, most included studies were retrospective cohort studies with the accompanying heterogeneity and higher risk of bias. Heterogeneity occurred due to different diagnostic approaches and variety in used definition of (hs)PDA. Conservative management in the included studies was predominantly classified as no treatment with indomethacin, ibuprofen, acetaminophen, and/or ligation. If specifically defined, it was highly variable from watchful waiting to the use of diuretics and/or fluid restriction and/or ventilator adjustments.
With the currently available literature regarding conservative PDA management, one might conclude that it appears safe to wait for delayed spontaneous closure based on RCT data. However, cohort studies suggest that conservative management is associated with a higher risk for mortality, but a lower risk for morbidity, albeit with a very low level of evidence. Therefore, a conservative management cannot be generalized to all preterm infants with a PDA and considered evidence-based practice at this moment.
Instead of dichotomizing a PDA as present or not, one should consider the PDA as a spectrum in which the amount of shunt volume across the PDA is thought to be associated with adverse outcome. To asses shunt volume (neonatologist performed), echocardiography could play an important role (38). Additional objective measurements indicative of transductal left-to-right shunt volume, rather than DA diameter alone, could better indicate hemodynamic significance, for example, the PDA severity score (39).
The high amount of active treatment in cohort studies and open-label treatment in RCTs suggests that in case of PDA associated morbidities clinicians might try to rule out a putative causal role of a PDA and therefor initiate active treatment in an attempt to achieve PDA closure. As included cohort studies mainly stratified patients regarding their final PDA treatment (“as treated”), instead of the initial management to which RCTs randomize (“intention to treat”), our meta-analysis could not correct for treatment selection bias, which is one of the main limitations.
The tendency of clinicians to actively close the DA in case of associated findings, hence absence of clinical equipoise, remains one of the main limitations in RCTs. In the PDA TOLERATE trial (40), 48% of the patients allocated to conservative management received open-label active treatment, referred to as “rescue” treatment (40). For future RCTs, we would suggest defining “open-label treatment” criteria as “rescue treatment” insinuates treatment is superior to conservative management in preterm infants with a PDA for which evidence is lacking. Together with the different types of bias in both RCTs and cohort studies rescue treatment contributes to the everlasting conundrum on PDA management.
In conclusion, we found no differences in outcome in the included RCTs. Our meta-analysis highlights the lack of high-quality evidence for conservative management for PDA in preterm infants.
The current trend toward conservative management cannot be justified based on these scarce, mainly retrospective and very heterogeneous cohort studies. Further cohort studies will not be able to provide a final and conclusive answer to the question whether we should consider a PDA in preterm infants as an epiphenomenon which can be managed conservatively or as an important causal factor or contributing factor to adverse outcome in preterm infants. High-quality RCTs with a conservative management group with a limited—preferably without—open-label treatment rate are needed to elucidate the conundrum whether or not to treat a PDA in extremely preterm infants.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
TH, EJ, and WB initiated the idea for this systematic review and meta-analysis. TH, WB, and WO contributed to the design of the study. Data acquisition and analysis were done by TH and EJ. WO, EK, PA, and WB revised the article critically for important intellectual content. All authors approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. R. Donders, biostatistician for his input on the statistical analysis plan of this systematic review and meta-analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.626261/full#supplementary-material
References
- 1.Lee JA, Kim MJ, Oh S, Choi BM. Current status of therapeutic strategies for patent ductus arteriosus in very-low-birth-weight infants in Korea. J Korean Med Sci. (2015) 30(Suppl. 1):S59–66. 10.3346/jkms.2015.30.S1.S59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks JM, Travadi JN, Patole SK, Doherty DA, Simmer K. Is surgical ligation of patent ductus arteriosus necessary? The Western Australian experience of conservative management. Arch Dis Child Fetal Neonatal Ed. (2005) 90:F235–9. 10.1136/adc.2004.057638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. (2010) 2010:CD000174. 10.1002/14651858.CD000174.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA, et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA. (2018) 319:1221–38. 10.1001/jama.2018.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zonnenberg I, de Waal K. The definition of a haemodynamic significant duct in randomized controlled trials: a systematic literature review. Acta Paediatr. (2012) 101:247–51. 10.1111/j.1651-2227.2011.02468.x [DOI] [PubMed] [Google Scholar]
- 6.Hundscheid T, Onland W, van Overmeire B, Dijk P, van Kaam A, Dijkman KP, et al. Early treatment versus expectative management of patent ductus arteriosus in preterm infants: a multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial). BMC Pediatr. (2018) 18:262. 10.1186/s12887-018-1215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirea L, Sankaran K, Seshia M, Ohlsson A, Allen AC, Aziz K, et al. Treatment of patent ductus arteriosus and neonatal mortality/morbidities: adjustment for treatment selection bias. J Pediatr. (2012) 161:689–94.e1. 10.1016/j.jpeds.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 8.Weisz DE, Mirea L, Resende MHF, Ly L, Church PT, Kelly E, et al. Outcomes of surgical ligation after unsuccessful pharmacotherapy for patent ductus arteriosus in neonates born extremely preterm. J Pediatr. (2018) 195:292–6 e3. 10.1016/j.jpeds.2017.11.029 [DOI] [PubMed] [Google Scholar]
- 9.Weisz DE, Mirea L, Rosenberg E, Jang M, Ly L, Church PT, et al. Association of patent ductus arteriosus ligation with death or neurodevelopmental impairment among extremely preterm infants. JAMA Pediatr. (2017) 171:443–9. 10.1001/jamapediatrics.2016.5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngo S, Profit J, Gould JB, Lee HC. Trends in patent ductus arteriosus diagnosis and management for very low birth weight infants. Pediatrics. (2017) 139:e20162390. 10.1542/peds.2016-2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farooqui MA, Elsayed Y, Jeyaraman MM, Dingwall O, Tagin M, Zarychanski R, et al. Pre-symptomatic targeted treatment of patent ductus arteriosus in preterm newborns: a systematic review and meta-analysis. J Neonatal Perinatal Med. (2018) 12:1–7. 10.3233/NPM-17130 [DOI] [PubMed] [Google Scholar]
- 12.Sung SI, Chang YS, Kim J, Choi JH, Ahn SY, Park WS. Natural evolution of ductus arteriosus with noninterventional conservative management in extremely preterm infants born at 23–28 weeks of gestation. PLoS ONE. (2019) 14:e0212256. 10.1371/journal.pone.0212256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semberova J, Sirc J, Miletin J, Kucera J, Berka I, Sebkova S, et al. Spontaneous closure of patent ductus arteriosus in infants ≤ 1500 g. Pediatrics. (2017) 140:e20164258. 10.1542/peds.2016-4258 [DOI] [PubMed] [Google Scholar]
- 14.Weber SC, Weiss K, Buhrer C, Hansmann G, Koehne P, Sallmon H. Natural history of patent ductus arteriosus in very low birth weight infants after discharge. J Pediatr. (2015) 167:1149–51. 10.1016/j.jpeds.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. (2000). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed June 1, 2020).
- 18.Serghiou S, Goodman SN. Random-effects meta-analysis: summarizing evidence with caveats. JAMA. (2019) 321:301–2. 10.1001/jama.2018.19684 [DOI] [PubMed] [Google Scholar]
- 19.GRADE . Handbook for Grading Quality of Evidence and Strength of Recommendations: The GRADE Working Group. (2013). Available online at: guidelinedevelopment.org/handbook (accessed June 1, 2020).
- 20.Harkin P, Harma A, Aikio O, Valkama M, Leskinen M, Saarela T, et al. Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. J Pediatr. (2016) 177:72–7 e2. 10.1016/j.jpeds.2016.04.066 [DOI] [PubMed] [Google Scholar]
- 21.Kumar Nair PA, Pai MG, Gazal HA, Da Costa DE, Al Khusaiby SM. Indomethacin prophylaxis for intraventricular hemorrhage in very low birth weight babies. Indian Pediatr. (2004) 41:551–8. [PubMed] [Google Scholar]
- 22.Sung SI, Lee MH, Ahn SY, Chang YS, Park WS. Effect of nonintervention vs oral ibuprofen in patent ductus arteriosus in preterm infants: a randomized clinical trial. JAMA Pediatr. (2020) 174:755–63. 10.1001/jamapediatrics.2020.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Overmeire B, Allegaert K, Casaer A, Debauche C, Decaluwe W, Jespers A, et al. Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. (2004) 364:1945–9. 10.1016/S0140-6736(04)17477-1 [DOI] [PubMed] [Google Scholar]
- 24.Alexander F, Chiu L, Kroh M, Hammel J, Moore J. Analysis of outcome in 298 extremely low-birth-weight infants with patent ductus arteriosus. J Pediatr Surg. (2009) 44:112–7. disscusion 7. 10.1016/j.jpedsurg.2008.10.019 [DOI] [PubMed] [Google Scholar]
- 25.Bourgoin L, Cipierre C, Hauet Q, Basset H, Gournay V, Roze JC, et al. Neurodevelopmental outcome at 2 years of age according to patent ductus arteriosus management in very preterm infants. Neonatology. (2016) 109:139–46. 10.1159/000442278 [DOI] [PubMed] [Google Scholar]
- 26.Harkin P, Marttila R, Pokka T, Saarela T, Hallman M. Morbidities associated with patent ductus arteriosus in preterm infants. Nationwide cohort study. J Matern Fetal Neonatal Med. (2018) 31:2576–83. 10.1080/14767058.2017.1347921 [DOI] [PubMed] [Google Scholar]
- 27.Heuchan AM, Young D. Early colour doppler duct diameter and symptomatic patent ductus arteriosus in a cyclo-oxygenase inhibitor naive population. Acta Paediatr, Int J Paediatr. (2013) 102:254–7. 10.1111/apa.12103 [DOI] [PubMed] [Google Scholar]
- 28.Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol. (2007) 27:164–70. 10.1038/sj.jp.7211662 [DOI] [PubMed] [Google Scholar]
- 29.Letshwiti JB, Semberova J, Pichova K, Dempsey EM, Franklin OM, Miletin J. A conservative treatment of patent ductus arteriosus in very low birth weight infants. Early Hum Dev. (2017) 104:45–9. 10.1016/j.earlhumdev.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 30.Lokku A, Mirea L, Lee SK, Shah PS. Trends and outcomes of patent ductus arteriosus treatment in very preterm infants in Canada. Am J Perinatol. (2017) 34:441–50. 10.1055/s-0036-1593351 [DOI] [PubMed] [Google Scholar]
- 31.Mohamed MA, El-Dib M, Alqahtani S, Alyami K, Ibrahim AN, Aly H. Patent ductus arteriosus in premature infants: to treat or not to treat? J Perinatol. (2017) 37:652–7. 10.1038/jp.2017.4 [DOI] [PubMed] [Google Scholar]
- 32.Sadeck LS, Leone CR, Procianoy RS, Guinsburg R, Marba ST, Martinez FE, et al. Effects of therapeutic approach on the neonatal evolution of very low birth weight infants with patent ductus arteriosus. J Pediatr. (2014) 90:616–23. 10.1016/j.jped.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 33.Slaughter JL, Reagan PB, Newman TB, Klebanoff MA. Comparative effectiveness of nonsteroidal anti-inflammatory drug treatment vs no treatment for patent ductus arteriosus in preterm infants. JAMA Pediatr. (2017) 171:e164354. 10.1001/jamapediatrics.2016.4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung SI, Chang YS, Chun JY, Yoon SA, Yoo HS, Ahn SY, et al. Mandatory closure versus nonintervention for patent ductus arteriosus in very preterm infants. J Pediatr. (2016) 177:66–71 e1. 10.1016/j.jpeds.2016.06.046 [DOI] [PubMed] [Google Scholar]
- 35.Isayama T, Mirea L, Mori R, Kusuda S, Fujimura M, Lee SK, et al. Patent ductus arteriosus management and outcomes in Japan and Canada: comparison of proactive and selective approaches. Am J Perinatol. (2015) 32:1087–94. 10.1055/s-0035-1548727 [DOI] [PubMed] [Google Scholar]
- 36.Clyman RI, Hills NK. The effect of prolonged tracheal intubation on the association between patent ductus arteriosus and bronchopulmonary dysplasia (grades 2 and 3). J Perinatol. (2020) 40:1358–65. 10.1038/s41372-020-0718-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. (2001) 344:1966–72. 10.1056/NEJM200106283442602 [DOI] [PubMed] [Google Scholar]
- 38.van Laere D, van Overmeire B, Gupta S, El-Khuffash A, Savoia M, McNamara PJ, et al. Application of NPE in the assessment of a patent ductus arteriosus. Pediatr Res. (2018) 84(Suppl. 1):46–56. 10.1038/s41390-018-0077-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Khuffash A, James AT, Corcoran JD, Dicker P, Franklin O, Elsayed YN, et al. A patent ductus arteriosus severity score predicts chronic lung disease or death before discharge. J Pediatr. (2015) 167:1354–61.e2. 10.1016/j.jpeds.2015.09.028 [DOI] [PubMed] [Google Scholar]
- 40.Clyman RI, Liebowitz M, Kaempf J, Erdeve O, Bulbul A, Hakansson S, et al. PDA-TOLERATE trial: an exploratory randomized controlled trial of treatment of moderate-to-large patent ductus arteriosus at 1 week of age. J Pediatr. (2018) 205:41–8.e6. 10.1016/j.jpeds.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.