Abstract
Purpose
Methylation of ingested inorganic arsenic (InAs) to monomethyl- (MMAs) and dimethyl-arsenical species (DMAs) facilitates urinary arsenic elimination. Folate and creatine supplementation influenced arsenic methylation in a randomized controlled trial (RCT). Here we examine if baseline status of one-carbon metabolism nutrients (folate, choline, betaine, and vitamin B12) modified the effects of FA and creatine supplementation on changes in homocysteine, guanidinoacetate (GAA), total blood arsenic, and urinary arsenic metabolite proportions and indices.
Methods
Study participants (N = 622) received 400 or 800 μg FA, 3 g creatine, 400 μg FA + 3 g creatine, or placebo daily for 12 weeks.
Results
Relative to placebo, FA supplementation was associated with greater mean increases in %DMAs among participants with betaine concentrations below the median than those with levels above the median (FDR < 0.05). 400 μg FA/day was associated with a greater decrease in homocysteine among participants with plasma folate concentrations below, compared with those above, the median (FDR < 0.03). Creatine treatment was associated with a significant decrease in %MMAs among participants with choline concentrations below the median (P = 0.04), but not among participants above the median (P = 0.94); this effect did not significantly differ between strata (P = 0.10).
Conclusion
Effects of FA and creatine supplementation on arsenic methylation capacity were greater among individuals with low betaine and choline status, respectively. The efficacy of FA and creatine interventions to facilitate arsenic methylation may be modified by choline and betaine nutritional status.
Clinical Trial Registry Identifier
NCT01050556, U.S. National Library of Medicine, https://clinicaltrials.gov; registered January 15, 2010.
Keywords: arsenic methylation, one-carbon metabolism, folic acid, creatine, choline, betaine
Introduction
Arsenic exposure through drinking water is a global public health concern. Over 140 million people in more than 70 countries, including 40 million people in Bangladesh [1], are exposed to arsenic concentrations > 10 μg/L, the World Health Organization (WHO) guideline [2, 3]. Chronic arsenic exposure has been associated with adverse health outcomes including cardiovascular disease, diabetes, skin lesions (melanosis, leukomelanosis, and keratosis), cancers (bladder, kidney, liver, lung, skin, and prostate), and impaired intellectual function [2, 4].
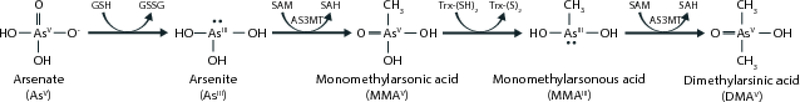
Methylation of inorganic arsenic (InAs) to mono- and di-methyl arsenical species facilitates urinary arsenic excretion [5, 6]. Ingested InAsIII is methylated to monomethylarsonic acid (MMAsV), reduced to MMAsIII, and methylated to dimethylarsinic acid (DMAsV) [7]. Arsenic methylation is catalyzed by arsenic-3-methyltransferase (AS3MT) using the methyl donor S-adenosylmethionine (SAM) [8] (Figure 1). Toxicological studies have demonstrated that MMAsIII is the most cytotoxic and genotoxic arsenic species [9, 10]. Although it is difficult to distinguish between MMAsIII and MMAsV in human studies due to rapid oxidation, a higher proportion of MMAsIII+V (%MMAs) and lower %DMAs in urine has been associated with increased risks for bladder, breast, lung, and skin cancers; skin lesions; peripheral vascular disease; and atherosclerosis [11, 12].
Figure 1.
Arsenic methylation. Arsenite (AsIII) is methylated to form monomethylarsonic acid (MMAsV) by arsenic methyltransferase (AS3MT) using the methyl donor S-adenosylmethionine (SAM). MMAsV is subsequently reduced to monomethylarsonous acid (MMAsIII) and methylated to form dimethylarsinic acid (DMAsV).
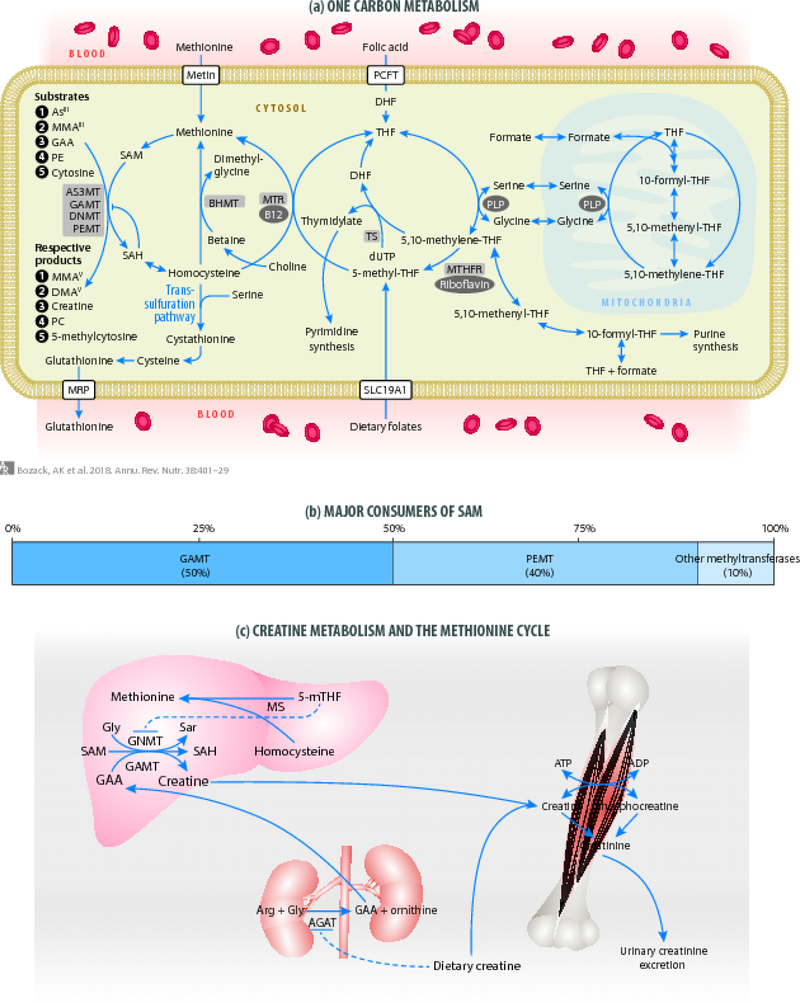
Arsenic metabolism efficiency varies between individuals and is influenced by one-carbon metabolism, the biochemical pathway that synthesizes SAM. Recruitment of one-carbon units into one-carbon metabolism is influenced by folate; one-carbon metabolism is also influenced by cofactors (e.g., vitamin B12) or alternative methyl donors (choline and betaine). A one-carbon unit is transferred from folate in the form of 5-methyl-tetrahydrofolate (5-methyl-THF) to homocysteine by methionine synthase using the cofactor vitamin B12 to form methionine, which is activated to SAM (Figure 2). Betaine, obtained through diet or synthesized from choline, can serve as an alternative methyl donor. When folate status is low, the use of betaine for homocysteine remethylation is increased [13].
Figure 2.
(a) One-carbon metabolism. Folic acid (FA) is reduced to dihydrofolate and tetrahydrofolate (THF) by dihydrofolate reductase. 5,10-methylene-THF is formed by serine hydroxymethyl-transferase through the transfer of one-carbon units from serine to THF, which is for thymidylate synthesis or reduced to 5-methyl-THF. Folate obtained through the diet can enter one-carbon metabolism as 5-methyl-THF. A one-carbon unit is transferred from 5-methyl-THF to homocysteine by methionine synthase using vitamin B12 as a cofactor to form methionine and THF. Homocysteine can also be remethylated in the liver by betaine homocysteine methyltransferase using betaine as the methyl donor. Methionine is activated to from S-adenosylmethionine (SAM) by methionine adenosyltransferase enzymes. SAM serves as the methyl donor for numerous reactions including arsenic methylation and biosynthesis of creatine, generating the methylated products and S-adenosylhomocysteine (SAH). SAH, which serves as a product inhibitor for most methyltransferase enzymes, hydrolyzed to homocysteine, and can either be remethylated to methionine or be directed towards the transsulfuration pathway. Adapted with permission from [15]. (b) Major consumers of SAM. An estimated 50% of SAM is consumed by the final step of endogenous creatine synthesis by GAMT, and 40% of SAM is consumed by phosphatidylcholine biosynthesis. (c) Creatine metabolism and the methionine cycle. In the kidney, arginine:glycine amidinotransferase (AGAT) produces guanidinoacetate (GAA). Dietary and/or supplemental creatine reduces GAA biosynthesis through the pretranslational inhibition of AGAT. GAA is released from the kidney and taken up by the liver where it is methylated using the methyl donor SAM to form creatine and SAH. SAH is hydrolyzed to homocysteine. 5-mTHF can regulate SAM and SAH levels through potent inhibition of GNMT. Creatine is transported to tissues and phosphorylated to phosphocreatine. Creatine and phosphocreatine are converted to creatinine and excreted in urine.
Dietary creatine may also influence the availability of SAM. An estimated 50% of SAM is consumed by creatine biosynthesis from guanidinoacetate (GAA) (Figure 2). Dietary sources, predominantly meat, provide approximately half of the daily requirement for creatine [14].
Our group and others have reported that dietary folate intake and folate status are positively associated with arsenic methylation capacity (reviewed in Bozack et al. [15]). We have also studied the effect of FA supplementation on arsenic metabolism and elimination in Bangladeshi adults. In a 12-week randomized controlled trial (RCT) among participants with plasma folate < 9 nmol/L, 400 μg FA/day supplementation was associated with a larger increase in urinary %DMAs and decreases in %InAs, %MMAs [16], total blood arsenic concentration, and blood MMAs concentration compared to placebo [17]. In the Folic Acid and Creatine Trial (FACT), an RCT among adults recruited independent of folate status, we observed a larger increase in urinary %DMAs and decreases in %InAs and %MMAs after 12 weeks of 400 or 800 μg FA/day supplementation [18], and a larger decrease in blood arsenic with 800 μg FA/day supplementation compared to placebo [19]. Supplementation with 400 and 800 μg FA resulted in significant increases in plasma betaine, illustrating the “sparing” effect of FA on betaine for homocysteine remethylation [20].
The associations between additional one-carbon metabolism micronutrients and arsenic methylation capacity have also been investigated [15]. Urinary creatinine, a product of creatine metabolism and a biomarker of dietary creatine intake and endogenous creatine biosynthesis, has been consistently associated with lower %InAs and higher %DMAs in urine in cross-sectional analyses [16, 18, 21–26]. In FACT, 3 g/day creatine was associated with a larger decrease in plasma GAA compared to placebo, indicating downregulation of endogenous creatine synthesis [27]. Creatine supplementation was associated with a larger decrease in urinary %MMAs compared to placebo at 6 and 12 weeks, but, surprisingly, was not associated with significant changes in %InAs or %DMAs [18]. The association between choline and betaine and arsenic methylation capacity has been investigated using food frequency questionnaire data. Dietary choline, but not betaine, has been positively associated with arsenic methylation capacity as measured by %InAs, %DMAs, DMAs/InAs [28] and DMAs/MMAs [28, 29] in urine. Findings regarding the association between vitamin B12 and the proportion of urinary arsenic metabolites are less consistent; results differ in the direction and significance of the associations across studies [28, 30, 31].
It is not known if treatment effects of FA and creatine are modified by baseline status of one-carbon metabolism micronutrients. Given the reciprocal use of folate vs. choline/betaine for the remethylation of homocysteine, we hypothesized that participants with low baseline levels of one-carbon metabolism micronutrients would experience greater treatment effects due to a limited supply of methyl donors prior to treatment. The objectives of the analyses presented here are to determine if baseline folate, choline, betaine, and vitamin B12 status modify the effects of FA and creatine supplementation on changes in homocysteine, GAA, blood arsenic concentration, and urinary arsenic metabolite proportions and methylation indices.
Methods
Subjects
FACT is a completed randomized, double-blind, placebo-controlled trial, which was designed to investigate the effects of FA and creatine supplementation on change in total blood arsenic, and has been described in detail previously by Peters et al. [19]. Participants were randomly recruited from the Health Effects of Arsenic Longitudinal Study (HEALS) [32], a cohort of over 30,000 adults in Araihazar, Bangladesh. Participants were eligible for FACT if they were drinking from a household well with arsenic concentration ≥ 50 μg/L for at least one year prior to enrollment. Participants were excluded if they were pregnant, taking nutritional supplements, or had proteinuria, renal disease, diabetes, gastrointestinal problems, or other health issues.
Study Design
A total of 622 participants were recruited based on power calculations for the main outcome of mean difference in change in blood arsenic concentrations between a treatment group and placebo. This sample size was determined to achieve 80% power at alpha = 0.05 to detect a moderate effect size (i.e., 0.45 SD) [19]. Participants were provided with READ-F arsenic removal filters (READ-F filter; Brota Services International, Bangladesh) and were encouraged to use the filters for all drinking and cooking water [33]. As previously described [19], participants were assigned to one of five treatment groups: 400 μg FA/day (referred to hereafter as 400FA; N = 156), 800 μg FA/day (800FA; N = 154), 3 g creatine/day (creatine; N = 104), 3 g creatine and 400 μg FA/day (creatine+400FA; N = 104), and placebo (N = 104). The FA doses of 400 and 800 μg/day were selected to meet and exceed the U.S. RDA; the creatine dose of 3 g/day was selected to exceed daily creatine loss (approximately 2 g for 70 kg 20–39 year-old males) [14], to be sufficient to downregulate endogenous creatine synthesis. Supplements were provided by Atrium Innovations, Inc. (Westmount, Quebec). All participants, field staff, laboratory technicians, and investigators, with the exception of the data management specialists, were blinded to the treatment during the study.
During the first 12-week phase, participants received daily supplements or a placebo; during the second 12-week phase, participants in the FA treatment groups were randomly assigned to continue their FA treatment (400FA: n=77; 800FA: n=77) or to receive a placebo (400FA/placebo: n=76; 800FA/placebo: n=74), and participants in the creatine and creatine+400FA groups received a placebo to maintain the study blind. The second phase was designed to investigate rebound of treatment effects following cessation of FA supplementation and therefore is not included in the analyses presented here.
Results regarding changes in blood arsenic [19] and urinary arsenic methylation [18] have previously been published. The current analyses utilized data from weeks 0–12 to investigate the a priori hypothesis that baseline nutritional status modifies the association between FA and/or creatine supplementation and changes in total blood arsenic, urinary arsenic metabolite proportions and indices, homocysteine, and GAA between baseline and week 12.
Ethics
The Columbia University Medical Center Institutional Review Board (protocol AAAC8618) and the Bangladesh Medical Research Council approved the study protocol. Informed consent was obtained by staff physicians in Bangladesh.
Field work
Field work was conducted in 2010–2012. Five pairs of field staff (an interviewer and physician) conducted recruitment and home visits to collect venous blood (baseline and weeks 12 and 24) or urine samples (baseline and weeks 1, 6, 12, 13, 18, and 24). During daily home visits, health workers observed or inquired about participant compliance in taking the pills. Pill counts were conducted at weeks 12 and 24. Compliance was high (range: 79.1–100%; median: 99.5%; interquartile range: 98.3–100.0%) and did not differ substantially between treatment groups [19].
Laboratory measures
Sample handling procedures and laboratory methods have previously been described in detail [19, 34]. Venous blood samples were collected in EDTA vacutainer tubes, stored at 4°C in IsoRack cool packs (Brinkmann Instruments; Riverview, FL). Urine samples were collected in 50-mL acid-washed polypropylene tubes and stored in portable coolers. Samples were transported to our Araihazar field clinic within 4 hours. Blood plasma was separated using centrifugation. Blood and urine samples were shipped to Columbia University on dry ice and stored at −80°C and −20°C, respectively.
Total blood arsenic was measured using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (PerkinElmer Elan DRC II; Waltham, MA; with an AS 93+ autosampler) (intra- and inter-assay CVs: 2.7% and 5.7%, respectively) [35]. Plasma folate and vitamin B12 were measured by radioimmunoassay (SimulTRAC-SNB, MP Biomedicals) (intra- and inter-assay CVs: 5% and 13% for plasma folate; 6% and 17% for vitamin B12).
High performance liquid chromatography (HPLC) with fluorescence detection was used to measure total plasma homocysteine [36] (intra- and inter-assay CVs: 5% and 7%) and plasma GAA [37] (intra- and inter-assay CVs: 8% and 9%). Plasma choline and betaine were measured using liquid chromatography–tandem mass spectrometry (LC-MS/MS) [38, 39] (intra- and inter-assay CVs: 2.2% and 5.8% for plasma choline; 2.5% and 5.6% for plasma betaine).
Urinary arsenobetaine, arsenocholine, arsenicIII, arsenicV, MMAs, and DMAs were separated by HPLC and measured by ICP-MS with dynamic reaction cell [40] (intra- and inter-assay CVs: 10.1% and 12.2% for arsenobetaine and arsenocholine; 2.7% and 4.7% for arsenicIII and arsenicV; 2.8% and 3.9% for MMAs; 0.6% and 1.3% for DMAs). The sum of trivalent and pentavalent forms of each arsenic metabolite are reported here, which is standard practice due to oxidation during storage. A refractometer was used to measure specific gravity.
Study sample
Eleven participants discontinued the study due to adverse events (n = 6; placebo: abdominal cramps; 400FA: hypertension; 800 FA: abdominal cramps, vertigo, bilateral hydronephrosis; creatine: vertigo), pregnancy (n = 3; 400FA, creatine, and creatine+400FA), and dropout (n=2; placebo and 400FA). Five participants were dropped due to a missing sample (Supplemental Figure S1, CONSORT flow diagram).
The current analyses used data from venous blood samples at baseline and week 12 and urinary arsenic metabolites at baseline, week 1, week 6 and week 12. A total of 605 participants were available for blood biomarker analyses stratified by baseline choline and betaine status, and 606 participants were available for analyses stratified by vitamin B12 and plasma folate (placebo: N = 101; 400FA: N = 152; 800FA: N = 149; creatine: N = 100 and 101, respectively; creatine+400FA: N = 103). GAA was measured in a subset of participants to evaluate the effect of creatine supplementation on GAA [27]; 400FA and 800FA groups were excluded from analyses of change in GAA.
Missing urine biomarkers or biomarkers associated with missing specific gravity data (N = 5) were excluded from analyses of changes in urinary arsenic metabolites. Specific gravity ≤ 1.001 is accepted to be outside of the normal range [41]. Values ≤ 1.001 for specific gravity (N = 48 at baseline; N = 46 at week 12) or %InAs (N = 1 at baseline; N = 5 at week 12) were also excluded [42]. A total of 511 participants were included in analyses of the change in urinary arsenic metabolites stratified by choline and betaine, and 512 participants were included in analyses stratified by vitamin B12 and plasma folate (placebo: N = 85; 400FA: N = 128; 800FA: N = 122; creatine: N = 87 and 88, respectively; creatine+400FA: N = 89).
Samples with urinary arsenicIII and arsenicV concentrations below the limit of detection (LOD) were replaced with LOD/2 (0.025 μg/L) (baseline arsenicIII N = 5; baseline arsenicV N = 3; week 12 arsenicIII N = 6; week 12 arsenicV N = 9). %InAs, %MMAs, and %DMAs in urine were calculated by dividing the concentration of each species by the sum of arsenicIII + arsenicV + MMAs + DMAs concentrations. The primary methylation index (PMI) and secondary methylation index (SMI) were also calculated (MMAs/InAs and DMAs /MMAs, respectively). Arsenobetaine and arsenocholine were excluded from these calculations, because they are thought to be non-toxic forms of arsenic from dietary sources [43].
Statistical analysis
Means and SDs were calculated for baseline characteristics. Participants were categorized as high or low choline, betaine, plasma folate, and vitamin B12 using a median cut-off point (choline: 11.4 nmol/mL; betaine: 43.6 nmol/mL; plasma folate: 13.5 nmol/L; vitamin B12: 214.9 pmol/L). Differences in baseline homocysteine, GAA, blood arsenic, and urinary arsenic metabolite proportions and indices between treatment groups within each nutrient stratum were assessed using the Kruskal-Wallis rank sum test.
The distributions of each outcome (i.e., within-person changes at week 12 in blood arsenic concentration, homocysteine concentration, and urinary arsenic metabolite proportions and indices) and baseline variables were examined. Blood arsenic, homocysteine, %InAs, and SMI had right skewed distribution and natural log-transformation was used to reduce the distribution skewness of the baseline and week 12 variables so that the within-person change met linear model assumptions. Levene’s test was used to check the linear model assumption of homoscedasticity.
Analyses of treatment group effects were performed by intent-to-treat. For each nutrient stratum, mean differences between treatment and placebo groups in 12-week within-person changes of each outcome were estimated using linear regression models. In the case of heteroscedasticity, standard errors and P-values were calculated by a heteroscedasticity-consistent covariance matrix estimation using the sandwich package in R [44]. Due to baseline treatment group differences in urinary arsenic metabolite proportions and arsenic methylation indices within the strata above- and below-median of choline, betaine, and folate, and differences in GAA within folate strata (data not shown), the models predicting within-person change in urinary arsenic metabolite proportions and arsenic methylation indices stratified by choline, betaine, and folate were adjusted for baseline arsenic metabolite proportions or arsenic methylation indices, respectively. Models predicting change in GAA stratified by folate were adjusted for baseline GAA. A Wald test was used to detect differences between strata (above vs. below median) in the parameter for treatment effect (referred to hereafter as test for difference). To adjust for multiple tests in detecting differences in treatment effects between strata, the Benjamini-Hochberg adjustment on P-values was used to control for the false discovery rate (FDR) [45].
We observed creatine treatment effects on the within-person changes in %MMAs at week 1 in unstratified analyses (previously reported in [18]), as well as creatine treatment effects at week 12 in the low choline strata. To further examine whether the observed treatment effects over 12 weeks stratified by choline status may also be present at weeks 1 or 6, linear models with repeated measures were used (N = 538 participants with data on change in arsenic metabolite proportions from baseline to weeks 1, 6, or 12). The natural-log transformation of %InAs at each time point was used. Changes in the proportions of each metabolite since baseline were calculated for weeks 1, 6, and 12. Models included control variables for baseline metabolite proportion, and predictors of treatment group, time categories and group-by-time interactions, which had coefficients indicating treatment group differences in mean within-person change since baseline. Model parameters were estimated using a generalized estimating equation approach to account for within-subject correlations in the repeated measures.
Analyses were performed using R version 3.2.2 (Vienna, Austria) [46] and SAS 9.4 (Cary, NC).
Results
Baseline participant characteristics are presented in Table 1. Participants had a mean age of 38 years (range: 24–55), and approximately half of participants were male (50.5%). The majority of participants were folate sufficient (≥ 9 nmol/L in plasma: 80.2%) and vitamin B12 sufficient (≥ 151 pmol/L: 75.9%).
Table 1:
Participant characteristics at baseline.
| Placebo (N=101) |
400FA (N=152) |
800FA (N=149) |
Creatine (N=101) |
Creatine+400FA (N = 103) |
|
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| Age (years) | 37.0 (32.0, 44.0) | 38.0 (33.0, 45.3) | 39.0 (31.0, 44.0) | 38.0 (30.0, 45.0) | 38.0 (31.5, 43.0) |
| Male (%) | 50.5 | 50.7 | 50.3 | 50.5 | 50.5 |
| Smoking ever (%) a | 24.8 | 24.0 | 29.5 | 28.7 | 30.1 |
| Betel nut use ever (%) a | 27.7 | 24.0 | 24.2 | 24.8 | 20.4 |
| Owns land (%) b | 46.5 | 50.7 | 48.3 | 47.5 | 43.1 |
| Body mass index (kg/ m2) c | 19.6 (18.2, 22.1) | 19.1 (18.2, 22.1) | 19.4 (18.2, 22.1) | 19.5 (18.2, 22.1) | 19.2 (18.2, 22.1) |
| Water As (μg/L) | 112.0 (73.6, 183.2) | 100.5 (73.6, 183.2) | 100 (73.6, 183.2) | 110.6 (73.6, 183.2) | 100.0 (73.6, 183.2) |
| Blood As (μg/L) | 8.6 (6.0, 11.8) | 8.4 (6.0, 11.8) | 8.8 (6.0, 11.8) | 8.8 (6, 11.8) | 9.5 (6, 11.8) |
| Urinary As (μg/L) d,e | 139.8 (93.0, 203.6) | 139.2 (93, 203.6) | 148.1 (93.0, 203.6) | 141.4 (93, 203.6) | 170.7 (93, 203.6) |
| Urinary %InAs f | 14.9 (11.7, 16.7) | 13.4 (11.7, 16.7) | 12.8 (11.7, 16.7) | 13.4 (11.7, 16.7) | 12.8 (11.7, 16.7) |
| Urinary %MMAs f | 12.7 (10.1, 15.5) | 12.3 (10.1, 15.5) | 12.2 (10.1, 15.5) | 13.1 (10.1, 15.5) | 11.9 (10.1, 15.5) |
| Urinary %DMAs f | 73.0 (68.7, 76.6) | 73.8 (68.7, 76.6) | 74 (68.7, 76.6) | 72.3 (68.7, 76.6) | 74.8 (68.7, 76.6) |
| Urinary primary methylation index d,f | 0.9 (0.7, 1.1) | 1.0 (0.7, 1.1) | 0.9 (0.7, 1.1) | 1.0 (0.7, 1.1) | 1.0 (0.7, 1.1) |
| Urinary secondary methylation index d,f | 5.7 (4.5, 7.5) | 6.1 (4.5, 7.5) | 5.9 (4.5, 7.5) | 5.6 (4.5, 7.5) | 6.3 (4.5, 7.5) |
| Urinary creatinine (mg/dL) f | 43.1 (30.6, 63.3) | 46.5 (30.6, 63.3) | 51.2 (30.6, 63.3) | 50 (30.6, 63.3) | 54.1 (30.6, 63.3) |
| Plasma folate (nmol/L) | 13.3 (9.5, 17.7) | 12.7 (9.5, 17.7) | 13.8 (9.5, 17.7) | 14.9 (9.5, 17.7) | 13.9 (9.5, 17.7) |
| Folate deficient (< 9 nmol/L in plasma) (%) | 21.8 | 23.7 | 18.1 | 13.9 | 20.4 |
| Plasma homocysteine (μmol/L) | 11.7 (9.2, 15.8) | 11 (9.2, 15.8) | 11.5 (9.2, 15.8) | 11.1 (9.2, 15.8) | 11.3 (9.2, 15.8) |
| Hyperhomocysteinemia (≥ 13 μmol/L) | 42.6 | 36.8 | 39.6 | 38.6 | 37.9 |
| Plasma vitamin B12 (pmol/L) | 215.9 (152.5, 279.7) | 215.9 (152.5, 279.7) | 215.1 (152.5, 279.7) | 223.3 (152.5, 279.7) | 200.7 (152.5, 279.7) |
| Vitamin B12 deficient (< 151 pmol/L) (%) | 24.8 | 24.3 | 26.2 | 19.8 | 24.3 |
| Choline (nmol/mL) g | 11.4 (9.9, 13.0) | 11.3 (9.9, 13.0) | 11.6 (9.9, 13.0) | 11.9 (9.9, 13.0) | 11.4 (9.9, 13.0) |
| Betaine (nmol/mL) g | 44.5 (33.3, 57.8) | 42.6 (33.3, 57.8) | 43.7 (33.3, 57.8) | 43.6 (33.3, 57.8) | 43.1 (33.3, 57.8) |
| Plasma GAA h | 2.0 (1.5, 2.5) | - | - | 1.8 (1.5, 2.5) | 1.9 (1.5, 2.5) |
400FA: N = 150.
Creatine+400FA: N = 102.
Placebo: N = 100; 400FA: N = 149; 800FA: N = 146; creatine: N = 98; creatine+400FA: N = 102.
Adjusted for specific gravity.
Placebo: N = 94; 400FA: N = 140; 800FA: N = 133; creatine: N = 93; creatine+400FA: N = 97.
Placebo: N = 94; 400FA: N = 140; 800FA: N = 133; creatine: N = 94; creatine+400FA: N = 97.
Creatine: N = 100.
Creatine+400FA: N = 102.
Treatment effects on homocysteine
There were significant differences in the change in homocysteine over 12 weeks between participants with baseline plasma folate below and above the median (Table 2). The mean within-person decrease in ln(homocysteine) relative to placebo was significantly greater in the low folate stratum with 400FA (low folate: B = −0.33, P < 0.001; high folate: B = −0.16, P < 0.001; test for difference between strata: P = 0.011, FDR = 0.024) and creatine+400FA supplementation (low folate: B = −0.31, P < 0.001; high folate: B = −0.14, P < 0.002; test for difference P = 0.012, FDR = 0.024). The difference between strata in the effects of 800FA on change in ln(homocysteine) was similar but with lower statistical significance (low folate: B = −0.34, P < 0.001; high folate: B = −0.20, P < 0.001; test for difference P = 0.049, FDR = 0.065).
Table 2:
Linear models for change in ln(homocysteine) over 12 weeks, by baseline plasma folate strata.a
| Change (week 12 - week 0) |
Low strata (≤ median) |
High strata (> median) |
Test for difference between strata b |
|||
|---|---|---|---|---|---|---|
| Treatment vs. placebo | B (95% CI) | P | B (95% CI) | P | P | FDR |
| 400FA | −0.33 (−0.43, −0.23) | <0.001 | −0.16 (−0.25, −0.08) | <0.001 | 0.011 | 0.024 |
| 800FA | −0.34 (−0.45, −0.23) | <0.001 | −0.20 (−0.29, −0.11) | <0.001 | 0.049 | 0.065 |
| Creatine | −0.05 (−0.15, 0.06) | 0.38 | −0.02 (−0.11, 0.07) | 0.67 | 0.70 | 0.70 |
| Creatine+400FA | −0.31 (−0.42, −0.21) | <0.001 | −0.14 (−0.22, −0.05) | 0.002 | 0.012 | 0.024 |
FDR = false discovery rate.
Placebo used as reference group. Baseline plasma folate median = 13.50 nmol/L.
Wald test for difference between low and high strata.
Treatment effects on arsenic methylation: FA
When stratifying by baseline betaine, mean within-person decreases in ln(%InAs) relative to placebo were greater among participants below the median with 400FA (low betaine: B = −0.19, P = 0.009; high betaine: B = −0.06, P = 0.28) and 800FA supplementation (low betaine: B = −0.29, P < 0.001; high betaine: B = −0.11, P = 0.035) (Table 3). The difference in treatment effects between strata with 400FA did not achieve statistical significance (P = 0.15); however, the difference between strata with 800FA was nominally significant (P = 0.04, FDR = 0.17). The decrease in %MMAs relative to placebo was greater among participants in the low betaine stratum with 400FA (low betaine: B = −3.06, P < 0.001; high betaine: B = −1.35, P = 0.01; test for difference P = 0.03, FDR = 0.10). Significantly greater mean within-person increases in %DMAs relative to placebo were also observed in the low betaine stratum with 400FA (low betaine: B = 6.02, P < 0.001; high betaine: B = 1.73, P = 0.07; test for difference P = 0.011, FDR = 0.044) and 800FA (low betaine: B = 7.07, P < 0.001; high betaine: B = 3.49, P <0.001; test for difference P = 0.022, FDR = 0.044). Differences in 400FA treatment effects between betaine strata were reflected in a greater mean within-person increase in ln(SMI) relative to placebo in the low stratum (B = 0.39, P < 0.001) compared with the high stratum (B = 0.14, P = 0.001) (test for difference P = 0.005; FDR = 0.021). Linear models with repeated measures indicated an increasing effect size of 400FA and 800FA on the change in arsenic metabolite proportions at weeks 1, 6, and 12 (Supplemental Table S2).
Table 3:
Linear models for change in As metabolite proportions over 12 weeks, by baseline betaine strata. a
| Low strata (≤ median) | High strata (> median) | Test for difference between strata b | |||||
|---|---|---|---|---|---|---|---|
| Change (week 12 – week 0) | Treatment vs. placebo | B (95% CI) | P | B (95% CI) | P | P | FDR |
| ln(%InAs) c | 400FA | −0.19 (−0.33, −0.05) | 0.009 | −0.06 (−0.17, 0.05) | 0.28 | 0.15 | 0.31 |
| 800FA | −0.29 (−0.43, −0.16) | <0.001 | −0.11 (−0.22, −0.01) | 0.035 | 0.042 | 0.17 | |
| Creatine | −0.02 (−0.15, 0.12) | 0.79 | −0.07 (−0.19, 0.05) | 0.26 | 0.59 | 0.79 | |
| Creatine+400FA | −0.15 (−0.32, 0.02) | 0.084 | −0.13 (−0.26, −0.01) | 0.035 | 0.88 | 0.88 | |
| %MMAs d | 400FA | −3.06 (−4.20, −1.92) | <0.001 | −1.35 (−2.33, −0.38) | 0.007 | 0.026 | 0.102 |
| 800FA | −2.93 (−4.02, −1.84) | <0.001 | −2.14 (−3.18, −1.11) | <0.001 | 0.30 | 0.40 | |
| Creatine | −1.09 (−2.44, 0.26) | 0.11 | −0.44 (−1.65, 0.77) | 0.48 | 0.48 | 0.48 | |
| Creatine+400FA | −2.78 (−4.02, −1.55) | <0.001 | −1.69 (−2.86, −0.51) | 0.005 | 0.21 | 0.40 | |
| %DMAs e | 400FA | 6.02 (3.29, 8.75) | <0.001 | 1.73 (−0.16, 3.62) | 0.073 | 0.011 | 0.044 |
| 800FA | 7.07 (4.62, 9.51) | <0.001 | 3.49 (1.63, 5.35) | <0.001 | 0.022 | 0.044 | |
| Creatine | 2.03 (−0.77, 4.83) | 0.16 | 1.03 (−1.15, 3.21) | 0.35 | 0.58 | 0.58 | |
| Creatine+400FA | 4.15 (0.91, 7.38) | 0.012 | 2.68 (0.54, 4.81) | 0.014 | 0.46 | 0.58 | |
FDR = false discovery rate. InAs = inorganic arsenic; MMAs = monomethyl-arsenical species; DMAs = dimethyl-arsenical species (DMAs PMI = primary methylation index measured in urine. SMI = secondary methylation index measured in urine.
Placebo used as reference group. Baseline betaine median = 43.63 nmol/mL.
Wald test for difference between low and high strata.
Adjusted for baseline ln(%InAs).
Adjusted for baseline %MMAs.
Adjusted for baseline %DMAs.
Treatment effects on arsenic methylation: Creatine
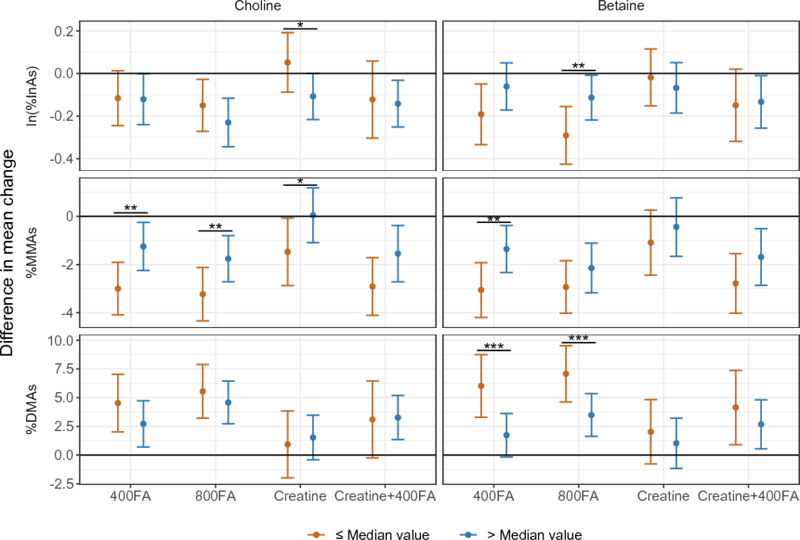
We observed differences in creatine treatment effects on the mean within-person changes in arsenic metabolite proportions over 12 weeks between participants above and below the median baseline choline (Table 4 and Figure 3). Creatine treatment led to a significant decrease in urinary %MMAs compared to placebo among participants in the low choline stratum (B = −1.47, P = 0.04), but not among participants in the high stratum (B = 0.05, P = 0.94), although the difference in the treatment effect was not statistically significant (test for difference P = 0.10). While there were no clear creatine effects on %InAs or %DMA, the three percentages are interrelated and changes in PMI and SMI were analyzed to evaluate overall direction of creatine treatment effects on arsenic methylation. There was a significant mean within-person decrease in PMI and increase in ln(SMI) among participants in the low choline stratum (PMI: B = −0.16, P = 0.035; ln(SMI): B = 0.14, P = 0.035), but not among participants in the high stratum (PMI: B = 0.06, P = 0.37; ln(SMI): B = 0.02, P = 0.69). Effect sizes for the change in PMI differed between strata, but the statistical significance was marginal after correcting for multiple tests (test for difference P = 0.028; FDR = 0.06). A similar pattern was observed when stratifying by betaine: the increase in ln(SMI) was significant in the low betaine stratum (B = 0.14, P = 0.033), but not in the high betaine stratum (B = 0.04, P = 0.46; test for difference P = 0.26) (Supplemental Table S1).
Table 4:
Linear models for change in As metabolite proportions over 12 weeks, by baseline choline strata. a
| Low strata (≤ median) | High strata (> median) | Test for difference between strata b | |||||
|---|---|---|---|---|---|---|---|
| Change (week 12 - week 0) | Treatment vs. placebo | B (95% CI) | P | B (95% CI) | P | P | FDR |
| ln(%InAs) c | 400FA | −0.12 (−0.24, 0.01) | 0.078 | −0.12 (−0.24, 0.00) | 0.048 | 0.96 | 0.96 |
| 800FA | −0.15 (−0.27, −0.03) | 0.017 | −0.23 (−0.34, −0.12) | <0.001 | 0.34 | 0.69 | |
| Creatine | 0.05 (−0.09, 0.19) | 0.47 | −0.11 (−0.22, 0.00) | 0.053 | 0.077 | 0.31 | |
| Creatine+400FA | −0.12 (−0.30, 0.06) | 0.19 | −0.14 (−0.25, −0.03) | 0.012 | 0.86 | 0.96 | |
| %MMAs d | 400FA | −3.00 (−4.09, −1.91) | <0.001 | −1.25 (−2.25, −0.25) | 0.014 | 0.020 | 0.078 |
| 800FA | −3.23 (−4.34, −2.12) | <0.001 | −1.76 (−2.72, −0.80) | <0.001 | 0.049 | 0.097 | |
| Creatine | −1.47 (−2.88, −0.07) | 0.040 | 0.05 (−1.09, 1.18) | 0.94 | 0.098 | 0.11 | |
| Creatine+400FA | −2.91 (−4.11, −1.72) | <0.001 | −1.55 (−2.72, −0.38) | 0.010 | 0.11 | 0.11 | |
| %DMAs e | 400FA | 4.53 (2.03, 7.04) | <0.001 | 2.71 (0.71, 4.72) | 0.008 | 0.27 | 0.93 |
| 800FA | 5.54 (3.21, 7.88) | <0.001 | 4.58 (2.72, 6.44) | <0.001 | 0.52 | 0.93 | |
| Creatine | 0.93 (−1.98, 3.83) | 0.53 | 1.53 (−0.41, 3.47) | 0.121 | 0.73 | 0.93 | |
| Creatine+400FA | 3.10 (−0.24, 6.44) | 0.069 | 3.27 (1.35, 5.19) | 0.001 | 0.93 | 0.93 | |
InAs = inorganic arsenic; MMAs = monomethyl−arsenical species; DMAs = dimethyl−arsenical species (DMAs
PMI = primary methylation index measured in urine. SMI = secondary methylation index measured in urine.
FDR = false discovery rate.
Placebo used as reference group. Baseline choline median = 11.42 nmol/mL.
Wald test for difference between low and high strata.
Adjusted for baseline ln(%InAs).
Adjusted for baseline %MMAs.
Adjusted for baseline %DMAs.
Figure 3.
Differences in mean change in urinary As metabolite proportions (week 12 – week 0) between treatment and placebo groups stratified by baseline choline and betaine below and above median. P-values are from Wald test for differences between strata in treatment effects based on linear models for within-person change in As metabolite proportions adjusting for baseline levels of As metabolite proportions, Baseline choline median = 11.42 nmol/mL; baseline betaine median = 43.63 nmol/mL. * Wald test for differences between strata P < 0.10; ** P < 0.05; *** FDR < 0.05.
In linear models with repeated measures, the mean within-person decrease in %MMAs was significantly greater in the creatine group than the placebo group among participants in the low choline stratum beginning one week after supplementation began (P = 0.028), and remained significantly greater at after 6 weeks (P = 0.003) and 12 weeks (P = 0.022) of supplementation (Supplemental Table S2). However, mean within-person changes in %MMAs were not significantly different between the creatine and placebo groups in the high choline stratum at any follow-up point.
Treatment effects on GAA: Creatine
The mean within-person decrease in ln(GAA) with creatine treatment relative to placebo was significant in the high choline and plasma folate strata (high choline: B = −0.21, P < 0.001; high folate: B = −0.22, P < 0.001) (Supplemental Table S1). The treatment effect was not significant in the low strata for either nutrient, although it was suggestive in the low folate stratum (low folate stratum: B = −0.10, P = 0.052). The difference in treatment effects by strata were marginally significant before correction for multiple tests (P = 0.083 for difference between choline strata; P = 0.091 for difference between folate strata).
Complete results for regression analyses stratified by baseline choline, betaine, vitamin B12, and plasma folate concentrations for all treatment groups are presented in Supplemental Table 1. We did not observe differences in the mean within-person change in blood arsenic with FA or creatine supplementation between the high and low strata of choline, betaine, vitamin B12, or plasma folate. In addition, the mean within-person changes in ln(homocysteine), blood arsenic, or urinary arsenic metabolite proportions with FA or creatine treatment relative to placebo did not differ by vitamin B12 strata.
Discussion
Arsenic methylation capacity is influenced by nutrients involved in one-carbon metabolism that affect the availability of one-carbon units, including folate, betaine, choline, and B12. This study investigated whether these nutrients modify FA and creatine treatment effects on changes in total homocysteine and GAA concentrations (biomarkers of one-carbon metabolism and endogenous creatine synthesis, respectively), total blood arsenic concentrations, and urinary arsenic metabolites.
Treatment effects on arsenic methylation
We observed that 400FA was associated with significant changes in urinary %InAs and %DMAs among participants in the low betaine stratum, but not among participants in the high betaine stratum. The mean within-person increase in %DMAs with 800FA was also greater among participants in the low betaine stratum compared with the high betaine stratum. These observations support our hypothesis that FA treatment effects would be greater among those with low betaine status due to the complementary role of folate for the remethylation of homocysteine under conditions of low betaine [47].
Urinary creatinine, a product of creatine metabolism, has been associated with arsenic methylation capacity in previous cross-sectional analyses [16, 18, 21–26], and in the current study at baseline. As previously reported, creatine supplementation was associated with a decrease in %MMAs among participants overall at weeks 1, 6 and 12 [18]. Here we find that, only among participants with choline concentrations below the median, creatine supplementation was associated with a decrease in %MMAs at week 1 that plateaus at weeks 6 through 12. Synthesis of phosphatidylcholine (PC), a precursor of choline, is a major consumer of SAM and producer of SAH[48] (Figure 2B), and is stimulated by low-choline diets[49, 50]. PC can be converted to choline and then betaine or used to satisfy other essential roles, such as lipid transport, cell signaling, and maintaining cell membranes. We speculate that low choline status, which upregulates PC synthesis, results in low SAM and high SAH, which may be reversed with creatine supplementation, allowing rapid methylation of MMAs to DMAs In support of this hypothesis, whole blood SAM concentrations were lower in the low choline stratum compared with the high choline stratum (t-test P = 0.017) and we observed significant decreases in PMI and increase in ln(SMI) among participants in the low choline stratum. The plateau in %MMAs at weeks 6 and 12 may be because the methylation of GAA occurs primarily in the liver [51], creatine treatment effects may be liver specific and this may be attenuated over time by long-range allosteric regulation of hepatic SAM. Possibly, the cross-sectional relationships between urinary creatinine and %InAs and %DMAs are due in part to renal tubular reabsorption of InAs under conditions of more concentrated urine [52] and/or may be related to dietary protein / methionine intake [53]. The relationships between creatine supplementation, urinary creatinine and arsenic methylation are likely complex and warrant further study.
Vitamin B12 deficiency limits the availability of one-carbon units for the synthesis of SAM [54], and we hypothesized that vitamin B12 status would modify treatment effects. However, associations between vitamin B12 and arsenic methylation capacity have been inconsistent [28, 30, 31], and we did not observe effect modification of FA or creatine treatment on the change in arsenic methylation by baseline vitamin B12 status.
In agreement with previous studies of the homocysteine-lowering effects of FA [55, 56], we observed a greater mean decrease in homocysteine concentration among participants with low plasma folate concentrations. However, we did not observe effect modification of FA or creatine treatment on the change in arsenic methylation by folate status, suggesting that FA supplementation enhances arsenic methylation capacity even among individuals with high folate status. This finding may also be due to elevated homocysteine in this population. At baseline, 63% of women and 72.9% of men had homocysteine levels above the normal range defined by the U.S. CDC (4.5–7.9 μmol/L and 6.3–11.2 μmol/L, respectively) [57]. Given this high prevalence of elevated homocysteine, FA treatment may lower homocysteine even among participants with plasma folate above the median.
Treatment effects on guanidinoacetate
Creatine supplementation downregulates creatine synthesis from GAA (Figure 2) [58]. As previously reported, creatine supplementation lowered GAA concentrations in the overall study population; as expected, FA did not affect GAA [27]. However, significant treatment effects of creatine in lowering GAA were observed in the high strata of choline and plasma folate but not in the low strata. The effects of creatine supplementation on lowering GAA may be most effective when there is both a reduction in GAA synthesis via inhibition of arginine:glycine amidinotransferase (AGAT) in the kidney and an increase in GAA methylation by guanidinoacetate methyltransferase (GAMT) in the liver. Mathematical models of OCM indicate that this can be facilitated by high folate, which inhibits glycine N-methyltransferase (GNMT, which otherwise regulates SAM concentrations) [59], allowing hepatic SAM concentrations to increase in response to creatine, and by a high choline diet, which reduces PEMT activity thus sparing SAM much in the same way as creatine supplementation, i.e., by reducing methyl demand [50].
Our study was limited in addressing the effect modification by of baseline status of folate, choline, betaine, and vitamin B12 due to data availability. We were not able to examine the effects of additional micronutrients involved in one-carbon metabolism, such as the cofactor vitamin B6. It should also be noted that sample size may have limited our power to identify effects when stratifying by baseline nutritional status. In addition, we selected the median baseline value to categorize participants as having high or low baseline nutritional status. This approach was reasonable for choline and betaine, micronutrients for which there are no reference ranges to determine deficiency, to maximize power. However, we were not able to determine if threshold effects could result in effect modification at different baseline micronutrient levels.
As observed in cross sectional studies, arsenic methylation capacity is associated with nutritional status related to one-carbon metabolism nutrients, including folate and creatine.[15] Although supplementation with SAM could also potentially increase As methylation, food fortification with SAM is not feasible, whereas fortification of grains with FA can nearly eradicate folate deficiency and is a potential public health intervention. This study contributes to an understanding of the relationships between FA and creatine supplementation and arsenic methylation capacity, and their dependence on nutritional status. Our group has previously reported the overall effects of FA and creatine supplementation on blood arsenic and arsenic methylation [16–19]. Here we observed that the effects of FA and creatine supplementation on arsenic methylation capacity were greater among individuals with low status of the alternative methyl donors, betaine and choline. These observations are relevant to arsenic-exposed individuals who may or may not be folate deficient but who have sub-optimal choline status, as also occurs in Western countries [60]. Although removal of arsenic from drinking water is the primary and most effective approach to decreasing arsenic-related morbidity and mortality [3], exposure remains a persistent public health concern in many regions of the world [2], including many regions where nutritional deficiencies are prevalent. Policies that aim to improve nutritional status may have the added benefit of reducing arsenic toxicity in arsenic-endemic regions.
Supplementary Material
Acknowledgements
We thank our staff, the fieldworkers, and the study participants in Bangladesh, without whom this work would not have been possible. This research was funded by National Institute of Environmental Health Sciences grants R00 ES018890, R01 CA133595, P30 ES009089, T32 ES007322, P42 ES010349, R01 ES017875, and F31 ES029019, and the National Institute of Health National Research Service Award TL1 TR001875. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations and their definitions
- MMAs
monomethyl-arsenical species
- DMAs
dimethyl-arsenical species
- RCT
randomized controlled trial
- FA
folic acid
- GAA
guanidinoacetate
- WHO
World Health Organization
- InAs
inorganic arsenic
- MMAsV
monomethylarsonic acid
- DMAsV
dimethylarsinic acid
- AS3MT
arsenic-3-methyltransferase
- SAM
S-adenosylmethionine: 5-methyl-THF: 5-methyl-tetrahydrofolate
- GAA
guanidinoacetate
- THF
tetrahydrofolate
- PC
phosphatidylcholine
- GAMT
guanidinoacetate methyltransferase
- SAM
S-adenosylhomocysteine
- RDA
recommended daily allowance
- FACT
Folic Acid and Creatine Trial
- HEALS
Health Effects of Arsenic Longitudinal Study
- ICP-MS
Inductively Coupled Plasma Mass Spectrometry
- HPLC
High performance liquid chromatography
- PMI
primary methylation index (PMI)
- SMI
secondary methylation index
- FDR
false discovery rate
- SAH
S-adenosylhomocysteine
- AGAT
arginine:glycine amidinotransferase
- GAMT
guanidinoacetate methyltransferase
- GNMT
glycine N-methyltransferase
Footnotes
Conflicts of interest/Competing interests: The authors declare that they have no conflicts of interest or competing interests.
Ethics approval: The Columbia University Medical Center Institutional Review Board (protocol AAAC8618) and the Bangladesh Medical Research Council approved the study protocol.
Consent to participate: Informed consent was obtained by staff physicians in Bangladesh.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Bangladesh Bureau of Statistics, United Nation Children’s Fund (2015) Bangladesh multiple indicator cluster survey 2012–2013 final report. Dhaka, Bangladesh [Google Scholar]
- 2.Naujokas MF, Anderson B, Ahsan H, et al. (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121:295–302. 10.1289/ehp.1205875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (2012) Arsenic. In: WHO Fact Sheets. http://www.who.int/mediacentre/factsheets/fs372/en/. Accessed 16 May 2016
- 4.Benbrahim-Tallaa L, Waalkes MP (2008) Inorganic arsenic and human prostate cancer. Environ Health Perspect 116:158–64. 10.1289/ehp.10423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tice RR, Yager JW, Andrews P, Crecelius E (1997) Effect of hepatic methyl donor status on urinary excretion and DNA damage in B6C3F1 mice treated with sodium arsenite. Mutat Res 386:315–34 [DOI] [PubMed] [Google Scholar]
- 6.Vahter M, Marafante E (1987) Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol Lett 37:41–6 [DOI] [PubMed] [Google Scholar]
- 7.Challenger F (1945) Biological methylation. Chem Rev 36:315–361. 10.1021/cr60115a003 [DOI] [Google Scholar]
- 8.Thomas D, Waters SB, Styblo M (2004) Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol 198:319–326. 10.1016/j.taap.2003.10.020 [DOI] [PubMed] [Google Scholar]
- 9.Petrick JS, Ayala-Fierro F, Cullen WR, et al. (2000) Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol 163:203–207. 10.1006/taap.1999.8872 [DOI] [PubMed] [Google Scholar]
- 10.Moe B, Peng H, Lu X, et al. (2016) Comparative cytotoxicity of fourteen trivalent and pentavalent arsenic species determined using real-time cell sensing. J Environ Sci 49:113–124. 10.1016/j.jes.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 11.Steinmaus C, Yuan Y, Kalman D, et al. (2010) Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol 247:138–45. 10.1016/j.taap.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo C-C, Moon KA, Wang S-L, et al. (2017) The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ Health Perspect 125:. 10.1289/EHP577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niculescu MD, Zeisel SH (2002) Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr 132:2333S–2335S. 10.1093/jn/132.8.2333S [DOI] [PubMed] [Google Scholar]
- 14.Brosnan JT, da Silva RP, Brosnan ME (2011) The metabolic burden of creatine synthesis. Amino Acids 40:1325–31. 10.1007/s00726-011-0853-y [DOI] [PubMed] [Google Scholar]
- 15.Bozack AK, Saxena R, Gamble MV (2018) Nutritional influences on one-carbon metabolism: effects on arsenic methylation and toxicity. Annu Rev Nutr 38:401–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamble MV, Liu X, Ahsan H, et al. (2006) Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid–supplementation trial in Bangladesh. Am J Clin Nutr 84:1093–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamble MV, Liu X, Slavkovich V, et al. (2007) Folic acid supplementation lowers blood arsenic. Am J Clin Nutr 86:1202–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozack AK, Hall MN, Liu X, et al. (2018) Folic acid supplementation enhances arsenic methylation: results from a folic acid and creatine supplementation randomized controlled trial in Bangladesh. Am J Clin Nutr 109:380–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters BA, Hall MN, Liu X, et al. (2015) Folic acid and creatine as therapeutic approaches to lower blood arsenic: a randomized controlled trial. Environ Health Perspect 123:1294–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall MN, Howe CG, Liu X, et al. (2016) Supplementation with folic acid, but not creatine, increases plasma betaine, decreases plasma dimethylglycine, and prevents a decrease in plasma choline in arsenic-exposed Bangladeshi adults. J Nutr 146:1062–1067. 10.3945/jn.115.227132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamble MV, Liu X, Ahsan H, et al. (2005) Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect 113:1683–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu A, Mitra S, Chung J, et al. (2011) Creatinine, diet, micronutrients, and arsenic methylation in West Bengal, India. Environ Health Perspect 119:1308–1313. 10.1289/ehp.1003393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kile ML, Hoffman E, Hsueh Y-M, et al. (2009) Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environ Health Perspect 117:455–460. 10.1289/ehp.11251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall M, Gamble M, Slavkovich V, et al. (2007) Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal–newborn pairs. Environ Health Perspect 115:1503–1509. 10.1289/ehp.9906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall MN, Liu X, Slavkovich V, et al. (2009) Folate, cobalamin, cysteine, homocysteine, and arsenic metabolism among children in Bangladesh. Environ Health Perspect 117:. 10.1289/ehp.0800164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilsner JR, Liu X, Ahsan H, et al. (2009) Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ Health Perspect 117:254–60. 10.1289/ehp.11872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters BA, Hall MN, Liu X, et al. (2015) Low-dose creatine supplementation lowers plasma guanidinoacetate, but not plasma homocysteine, in a double-blind, randomized, placebo-controlled trial. J Nutr 145:2245–52. 10.3945/jn.115.216739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Carrillo L, Gamboa-Loira B, Becerra W, et al. (2016) Dietary micronutrient intake and its relationship with arsenic metabolism in Mexican women. Environ Res 151:445–450. 10.1016/j.envres.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heck JE, Gamble MV, Chen Y, et al. (2007) Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr 85:1367–74 [DOI] [PubMed] [Google Scholar]
- 30.Hall M, Liu X, Slavkovich V, et al. (2009) Influence of cobalamin on arsenic metabolism in Bangladesh. Environ Health Perspect 117:1724–9. 10.1289/ehp.0900734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spratlen MJ, Gamble MV., Grau-Perez M, et al. (2017) Arsenic metabolism and one-carbon metabolism at low-moderate arsenic exposure: evidence from the Strong Heart Study. Food Chem Toxicol 105:387–397. 10.1016/j.fct.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahsan H, Chen Y, Parvez F, et al. (2006) Health Effects of Arsenic Longitudinal Study (HEALS): Description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol 16:191–205. 10.1038/sj.jea.7500449 [DOI] [PubMed] [Google Scholar]
- 33.Sanchez TR, Levy D, Shahriar MH, et al. (2016) Provision of well-water treatment units to 600 households in Bangladesh: a longitudinal analysis of urinary arsenic indicates fading utility. Sci Total Environ 563:131–137. 10.1016/j.scitotenv.2016.04.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howe CG, Liu X, Hall MN, et al. (2017) Sex-specific associations between one-carbon metabolism indices and posttranslational histone modifications in arsenic-exposed Bangladeshi adults. Cancer Epidemiol Biomarkers Prev 26:261–269. 10.1158/1055-9965.EPI-16-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall M, Chen Y, Ahsan H, et al. (2006) Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology 225:225–33. 10.1016/j.tox.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 36.Pfeiffer CM, Huff DL, Gunter EW (1999) Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 45:290–2 [PubMed] [Google Scholar]
- 37.Carducci C, Birarelli M, Leuzzi V, et al. (2002) Guanidinoacetate and creatine plus creatinine assessment in physiologic fluids: an effective diagnostic tool for the biochemical diagnosis of arginine:glycine amidinotransferase and guanidinoacetate methyltransferase deficiencies. Clin Chem 48:1772–8 [PubMed] [Google Scholar]
- 38.Holm PI, Ueland PM, Kvalheim G, Lien EA (2003) Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 49:286–94. 10.1373/49.2.286 [DOI] [PubMed] [Google Scholar]
- 39.Yan J, Jiang X, West AA, et al. (2012) Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr 95:1060–1071. 10.3945/ajcn.111.022772 [DOI] [PubMed] [Google Scholar]
- 40.Reuter W, Davidowski L, Neubauer K (2003) Speciation of five arsenic compounds in urine by HPLC/ICP-MS, PerkinElmerSCIEX
- 41.Vahter ME, Li L, Nermell B, et al. (2006) Arsenic exposure in pregnancy: a population-based study in Matlab, Bangladesh. J Health Popul Nutr 24:236–45 [PubMed] [Google Scholar]
- 42.Vahter M (1999) Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog 82 ( Pt 1):69–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syracuse Research Corporation (2007) Toxicological Profile for Arsenic (Update). Atlanta, GA [Google Scholar]
- 44.Zeileis A (2004) Econometric computing with HC and HAC covariance matrix estimators. J Stat Softw 11:1–17 [Google Scholar]
- 45.Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300 [Google Scholar]
- 46.R Core Team (2015) R: A Language and Environment for Statistical Computing. Vienna, Austria [Google Scholar]
- 47.Obeid R (2013) The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 5:3481–95. 10.3390/nu5093481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mudd H, Brosnan JT, Brosnan ME, et al. (2007) Methyl balance and transmethylation fluxes in humans. Am J Clin Nutr 85:19–25 [DOI] [PubMed] [Google Scholar]
- 49.Kennedy EP (1957) Metabolism of Lipides. Annu Rev Biochem 26:119–148. 10.1146/annurev.bi.26.070157.001003 [DOI] [PubMed] [Google Scholar]
- 50.Cui Z, Vance DE (1996) Expression of phosphatidylethanolamine N-methyltransferase-2 is markedly enhanced in long term choline-deficient rats. J Biol Chem 271:2839–2843. 10.1074/jbc.271.5.2839 [DOI] [PubMed] [Google Scholar]
- 51.Brosnan JT, Brosnan ME (2007) Creatine: Endogenous Metabolite, Dietary, and Therapeutic Supplement. Annu Rev Nutr 27:241–261. 10.1146/annurev.nutr.27.061406.093621 [DOI] [PubMed] [Google Scholar]
- 52.Ginsburg JM, Lotspeich WD (1963) Interrelations of arsenate and phosphate transport in the dog kidney. Am J Physiol 205:707–714. 10.1152/ajplegacy.1963.205.4.707 [DOI] [PubMed] [Google Scholar]
- 53.Steinmaus C, Carrigan K, Kalman D, et al. (2005) Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect 113:1153–9. 10.1289/ehp.7907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green R, Allen LH, Bjørke-Monsen A-L, et al. (2017) Vitamin B12 deficiency. Nat Rev Dis Prim 3:17040. 10.1038/nrdp.2017.40 [DOI] [PubMed] [Google Scholar]
- 55.Homocysteine Lowering Trialists’ Collaboration (1998) Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ 316:894–8. 10.1136/BMJ.316.7135.894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Homocysteine Lowering Trialists’ Collaboration (2005) Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr 82:806–812. 10.1093/ajcn/82.4.806 [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention (2003) Laboratory Procedure Manual: Total Homocysteine (tHcy), NHANES 2003–2004. Atlanta, GA [Google Scholar]
- 58.McGuire DM, Gross MD, Van Pilsum JF, Towle HC (1984) Repression of rat kidney L-arginine:glycine amidinotransferase synthesis by creatine at a pretranslational level. J Biol Chem 259:12034–8 [PubMed] [Google Scholar]
- 59.Reed MC, Gamble MV, Hall MN, Nijhout HF (2015) Mathematical analysis of the regulation of competing methyltransferases. BMC Syst Biol 9:. 10.1186/s12918-015-0215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeisel SH, da Costa K-A (2009) Choline: an essential nutrient for public health. Nutr Rev 67:615–23. 10.1111/j.1753-4887.2009.00246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.