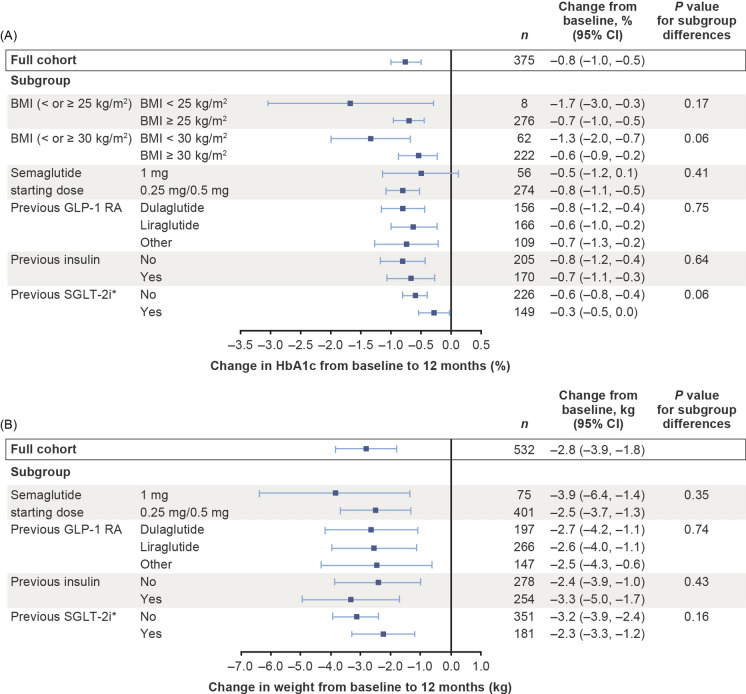
Fig. 6.
Changes in HbA1c (a) and weight (b) from baseline to 12 months in subgroups defined by baseline BMI, starting dose of semaglutide, baseline GLP-1 RA and previous treatment. Asterisk indicates that the minimal model was used owing to small available sample size. Changes from baseline in the full cohort using the minimal model were − 0.5% for HbA1c and − 2.9 kg for weight. Explanatory variables were subgroup, region, sex, age at index date, baseline weight, baseline HbA1c, Charlson–Deyo risk index score, cardiovascular disease in the past year, index year, insurance type, previous receipt of oral antidiabetic medications, time from actual follow-up assessments to nominal follow-up time and time from actual follow-up assessments to nominal follow-up time squared. Estimated treatment effects and corresponding 95% CIs were based on all patients with at least one prescription of the specific GLP-1 RA in the baseline period. When comparing subgroups by means of the P value, patients with prescriptions for different types of GLP-1 RAs in the baseline period were excluded. BMI body mass index, CI confidence interval, GLP-1 RA glucagon-like peptide-1 receptor agonist, HbA1c glycated haemoglobin, SGLT-2i sodium-glucose co-transporter-2 inhibitor