Abstract
Background
Yes-associated protein 1 (YAP1) is a transcription factor regulated by the Hippo pathway and functions as an oncogene in various solid tumors under dysregulated Hippo pathway. However, the role of YAP1 in breast cancer remains controversial. Here, we investigated the impact of different levels of nuclear YAP1 expression on the clinical characteristics and survival outcome in patients with breast cancer.
Patients and Methods
Retrospectively obtained 455 breast tumor samples at Gangnam Severance Hospital were examined for YAP1 expression by immunohistochemistry, and the clinical data were analyzed. External validation was performed using a retrospective cohort and tissues in 482 patients from Severance Hospital.
Results
High nuclear YAP1 expression was associated with hormone receptor negativity and aggressive tumor behavior, including lymph node metastasis, high Ki67 labeling index and inferior distant metastasis-free survival (DMFS, hazard ratio [HR] 2.271, 95% confidence intervals [CIs] 1.109–4.650, P = 0.0249), and also confirmed inferior disease free survival (HR 3.208, 95% CIs 1.313–7.833, P = 0.0105) in external validation cohort. In patients with triple-negative breast cancer (TNBC), high nuclear YAP1 expression was an independent significant determinant of poor DMFS (HR 2.384, 95% CIs 1.055–5.386, P = 0.0367).
Conclusion
Our findings suggest that nuclear YAP1 expression is a biomarker of adverse prognosis and a potential therapeutic target in patients with breast cancer, especially in TNBC.
Keywords: Yes-associated protein 1, breast cancer, prognosis, metastasis, triple-negative breast cancer
Background
Breast cancer is the most common cancer among women worldwide, and approximately 15 million people are diagnosed with this disease each year. In recent years, owing to advanced treatment modalities and the identification of new drug targets, breast cancer mortality has decreased by approximately 2.3% per year (1). So far, well known biomarkers such as including ER and HER2, as well as others, including pSTAT3 expression, LDH, and tumor-infiltrating lymphocytes, etc. – have been studied to elucidate breast cancer biology and survival outcome (2–4), further endeavor is still required to understand tumor nature. Despite these improvements, metastatic breast cancer still results in poor survival. In order to further enhance treatment efficacy, new drug targets with specific roles in metastatic cascades must be unveiled.
Lymph node metastasis is the most important prognostic factor in breast cancer, and is associated with high relapse and mortality (5–8). The mechanisms underlying lymph node metastasis are still poorly understood. It has been reported that the transcriptional co-activator, Yes-associated protein 1 (YAP1), plays an important role in lymph node metastasis (9). Enhanced YAP1 activity increases fatty acid oxidation, ultimately leading to lymph node metastasis. The Hippo signaling regulates organ size and development (10), and restricts transcription co-factor YAP1 and transcriptional coactivator with PDZ-binding motif (TAZ) by cytoplasmic retention followed by protein degradation (11). However, dysregulated Hippo signaling results in the nuclear accumulation of non-phosphorylated YAP1 and TAZ. These interact with transcriptional enhanced associated domain (TEAD)-containing transcription factors in nucleus, promoting the expression of genes related to cell proliferation and epithelial-mesenchymal transition (EMT) (12, 13). Moreover, YAP1 was reported to induce TEAD-dependent focal adhesion kinase phosphorylation, ultimately promoting tumor invasiveness (14). Activated YAP1 contributes to cancer development by promoting a malignant tumor phenotype. In particular, YAP1 stimulates cancer stem cell proliferation and epithelial-mesenchymal transition, induces drug resistance, inhibits apoptosis, and promotes tumor overgrowth (10, 15–18). Several studies have reported a correlation of YAP1 expression with aggressive clinical characteristics and low survival (19–29). This evidence suggests that YAP1 is a potential therapeutic target, and that its pharmacologic or genetic inactivation may suppress tumor progression and improve drug sensitivity. However, the association between clinical data and YAP1 expression in patients with breast cancer has been poorly explored. In addition, the possible clinical relevance of YAP1 subcellular localization is not clearly defined.
The aim of this study was to correlation between the level of nuclear YAP1 expression and the clinical characteristics and survival rates of patients with breast cancer. The impact of YAP1 expression on survival was also evaluated in patients with triple negative breast cancer (TNBC).
Materials and Methods
Patients
We retrospectively collected the tumor tissues from the patients undergoing primary curative surgery for breast cancer at the Gangnam Severance Hospital in Seoul, Korea from February 1992 to April 2017 and at the Severance Hospital in Seoul, Korea from January 2000 to December 2010. A validation cohort consisted of 482 patients from the Severance Hospital. Inclusion and exclusion criteria were as follows:
-
1) Inclusion criteria:
- Patients age ≥ 20 years
- Breast cancer confirmed by pathologic diagnosis (stage I–III)
- Available YAP1 immunohistochemical staining with tissue microarray
-
2) Exclusion criteria:
- Any other carcinoma in situ
- Other cancer history (except thyroid cancer and carcinoma in situ)
- Not assessable electronic medical record
All subjects were diagnosed with stage I–III primary breast cancer. All patient treatments were performed according to standard protocols. The following data were collected: age at surgery, tumor size, lymph node status, histological grade (HG), status of estrogen receptor (ER), status of progesterone receptor (PR), status of human epidermal growth factor receptor-2 (HER2), lymphovascular invasion (LVI), Ki67 leveling index, tumor-infiltrating lymphocytes (TILs), treatment modalities, recurrence, and death. Tumor HG was determined by applying the modified Scarff-Bloom-Richardson grading system. The study protocol was approved by the institutional review board (IRB) of the Gangnam Severance Hospital (local IRB No. 3-2019-0188). The need for informed consent was waived under the approval of the IRB due to the retrospective design.
Immunohistochemistry (IHC) and Molecular Subtyping
As previously described (30), 3-µm thick tissue sections were cut from formalin-fixed paraffin-embedded (FFPE) tissue microarray (TMA) blocks. After deparaffinization and rehydration with xylene and alcohol graded solutions, respectively, IHC was performed by using a Ventana Discovery XT Automated Slide Stainer (Ventana Medical System, Tucson, AZ, USA). Cell Conditioning 1 (CC1) buffer (citrate buffer, pH 6.0; Ventana Medical System) was used for antigen retrieval. The appropriate positive and negative controls were included.
IHC staining was evaluated with light microscopy (BX53 upright microscope, Olympus, Tokyo, Japan). Nuclear staining values of 1% or higher were considered indicative of ER (clone SP1; dilution 1:100; Thermo Scientific, San Diego, CA, USA) and PR (clone PgR; dilution 1:50; DAKO, Glostrup, Denmark) positivity (31). HER2 (polyclonal; dilution 1:1500; DAKO) staining was interpreted based on the 2018 American Society of Clinical Oncology/College of American Pathologists guidelines (32). Only samples with strong and circumferential membranous HER2 immunoreactivity (3+) were considered positive, while those with 0 and 1+ HER2 staining were considered negative. Cases with equivocal HER2 expression (2+) were further evaluated for HER-2 gene amplification by silver in situ hybridization (SISH). Positive nuclear Ki-67 (clone MIB; dilution 1:1,000; Abcam, Cambridge, UK) staining was assessed based on the percentage of positive tumor cells, defined as Ki-67 labeling index.
The specimens were categorized as follows: i) Luminal/HER2-negative (ER- and/or PR-positive and HER2-negative); ii) HER2-positive (HER2-positive regardless of ER and PR status); iii) TNBC (ER-, PR-, and HER2-negative).
Evaluation of Nuclear YAP1 Expression by Tissue Microarray and IHC Staining
Hematoxylin and eosin-stained slides from the resected breast cancer specimens were examined, and the representative areas were marked. The matched tissue cores (2 mm) were extracted from FFPE tumor blocks and placed into 5 × 10 recipient TMA blocks.
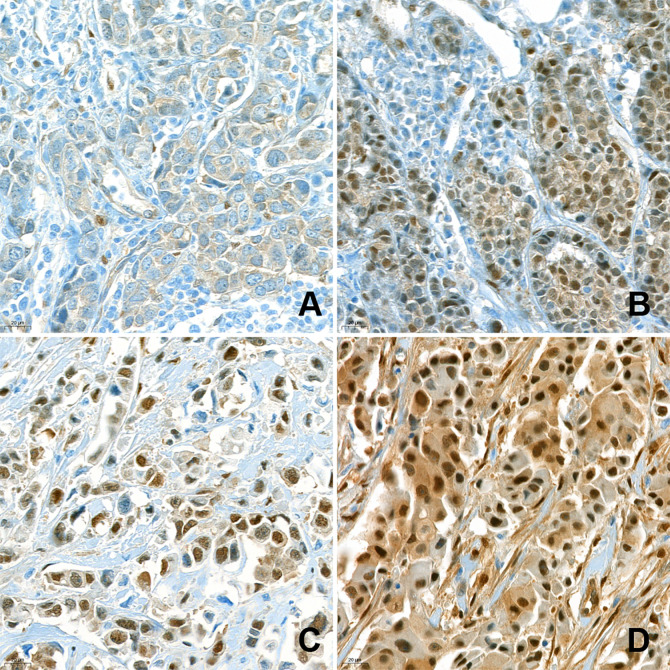
For IHC, each TMA slide was stained with anti-YAP1 antibody (clone 63.7; dilution 1:200; Santa Cruz Biotechnology, Dallas, TX, USA). After staining, nuclear YAP1 expression was assessed by a pathologist (400× magnification). YAP1 expression was evaluated in both the cytoplasm and nuclei of the tumor cells. Cytoplasmic staining was evaluated by the H-score, which was obtained by multiplying staining intensity (0, 1, 2, or 3) by percentage of stained area (%). As nuclear expression was rare and mostly focal, only the intensity of nuclear staining was examined (0, 1+, 2+, 3+), regardless of the corresponding cytoplasmic staining. The intensity of nuclear staining of the myoepithelial cells was assigned a value of 2+ and used as an internal control. Weaker and stronger signals were assigned a value of 1+ and 3+, respectively. Negative and weak (1+) nuclear staining were considered indicative of low expression, while moderate (2+) and strong (3+) nuclear expression were indicative of high expression ( Figure 1 ). The IHC results were interpreted blindly, without any information regarding clinical parameters or outcomes.
Figure 1.
Immunohistochemical analysis of nuclear YAP1 expression. Nuclear YAP1 expression was evaluated in high-power fields (400× magnification) by an experienced pathologist. The samples were classified as negative (A), 1+ (B), 2+ (C), and 3+ (D), based on the intensity of YAP1 nuclear staining.
Statistical Analysis
Distant metastasis-free survival (DMFS) was defined as the time from the primary curative surgery to the first breast cancer-derived distant metastasis, or death due to any cause, or end of follow-up. Overall survival (OS) was defined as the time from the primary curative surgery to the end of follow-up, or death due to any cause. Disease-free survival (DFS) was defined as the time from the primary curative surgery to cancer recurrence, second cancer, or death. The data of patients who did not exhibit relevant events were censored at the end of follow-up.
The continuous variables between the two groups were compared using the Student’s t-test or the Mann-Whitney test. The categorical variables were compared by using the Chi-square test or the Fisher’s exact test. Survival curves were obtained by the Kaplan-Meier method and two-group comparisons were made using log-rank test. Univariate and multivariate Cox proportional hazard models were used to identify the factors associated with survival outcome (DMFS and OS). The variables showing statistically significant differences in the univariate analysis were used in the multivariate Cox proportional hazard models.
Statistical analysis was performed by using SPSS version 24 (SPSS: Chicago, IL, USA) software. The threshold for statistical significance was set at P <0.05, with a 95% confidence interval not including 1.
Results
Impact of Nuclear YAP1 Expression on the Baseline Characteristics of Patients With Breast Cancer
A total of 455 breast cancer patients at Gangnam Severance Hospital were included in this study. The median age was 50 (25–86) years. The median DFS and OS were 59 (10–325) and 60 (12–325) months. Low and high nuclear YAP1 expression were found in the tumors of 344 (75.6%) and 111 (24.4%) patients, respectively. The clinical characteristics were examined in relation to nuclear YAP1 expression ( Table 1 ). High nuclear YAP1 expression was associated with aggressive tumor features, including hormone receptor negativity, high HG, lymph node metastasis, and high Ki67 expression. Patients were classified into three subtypes based on IHC analysis: luminal/HER2-negative (243 patients), HER2-positive (62 patients), and TNBC (146 patients). High nuclear YAP1 expression was associated with the TNBC subtype.
Table 1.
Clinical characteristics in relation to nuclear YAP1 expression.
| YAP1-low, n = 344 (%) | YAP1-high, n = 111 (%) | P value | |
|---|---|---|---|
| Age (year, mean ± SD) | 50.78 ± 10.47 | 49.51 ± 9.50 | 0.256 |
| HR | <0.001 | ||
| Positive | 238 (69.2) | 35 (31.5) | |
| Negative | 106 (30.8) | 76 (68.5) | |
| HER2 a | 0.301 | ||
| Positive | 50 (14.5) | 12 (10.8) | |
| Negative | 290 (84.3) | 99 (89.2) | |
| Missing | 4 (1.2) | 0 | |
| HG a | 0.005 | ||
| I, II | 223 (64.8) | 54 (48.6) | |
| III | 115 (33.4) | 52 (46.8) | |
| Missing | 6 (1.7) | 5 (4.5) | |
| Subtype a | <0.001 | ||
| Luminal/HER2(−) | 210 (61.0) | 33 (29.7) | |
| HER2 (+) | 50 (14.5) | 12 (10.8) | |
| TNBC | 80 (23.3) | 66 (59.9) | |
| Missing | 4 (1.2) | 0 | |
| Tumor size a | 0.298 | ||
| ≤2 cm | 174 (50.6) | 50 (45.0) | |
| >2 cm | 169 (49.1) | 61 (55.0) | |
| Missing | 1 (0.3) | 0 | |
| Lymph node metastasis a | 0.602 | ||
| Negative | 231 (67.2) | 72 (64.9) | |
| Positive | 111 (32.3) | 39 (35.1) | |
| Missing | 2 (0.6) | 0 | |
| Ki67 (%) a | 0.001 | ||
| ≤20% | 258 (75.0) | 62 (55.9) | |
| >20% | 74 (21.5) | 40 (36.0) | |
| Missing | 12 (3.5) | 9 (8.1) | |
| Lymphovascular invasion a | 0.413 | ||
| Negative | 268 (77.9) | 81 (73.0) | |
| Positive | 55 (16.0) | 21 (18.9) | |
| Missing | 21 (6.1)81 | 9 (8.1) | |
| TILs (%, mean ± SD) a | 33.84 ± 26.15 (n = 164) | 33.54 ± 30.77 (n = 72) | 0.943 |
SD, standard deviation; HR, hormone receptor; HER-2, human epidermal growth factor receptor-2; HG, histological grade; TNBC, triple negative breast cancer; TILs, tumor-infiltrating lymphocytes.
Percentages calculated without missing values.
Validation cohort included 482 patients at Severance Hospital. Median DFS and OS were 65 (5–139) and 65 (12–241) months. Of the 482 patients, 428 (88.8%) exhibited low nuclear YAP1 expression, and 54 (11.2%) exhibited high nuclear YAP1 expression. Clinical characteristics were compared to nuclear YAP1 expression in Supplementary Table 1 . Also, high nuclear YAP1 expression was related to TNBC subtype.
Prognostic Significance of Nuclear YAP1 Expression
There were 41 patients with developing distant metastasis. Among them, 14 had bone metastasis, 11 lung metastasis, five liver metastasis, three brain metastasis, while 17 had developed metastases to other sites (including duplication). There were 18 mortality events. High nuclear YAP1 expression was significantly associated with decreased distant metastasis-free survival (DMSF) [ Figure 2A ; hazard ratio (HR), 2.271, 95% confidence intervals (CIs) 1.109–4.650, P = 0.0249, log rank test], and was a significant predictor of poor overall survival ( Figure 2B ; HR 3.856, 95% CIs 1.321–11.26, P = 0.0135, log rank test).
Figure 2.
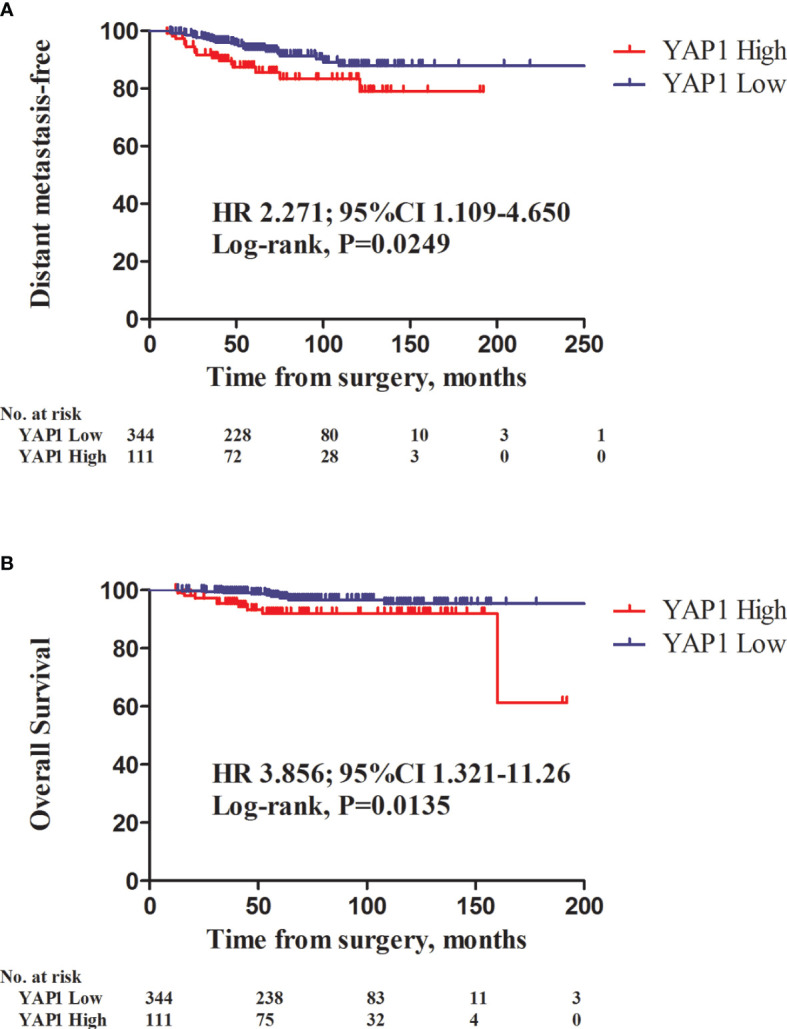
Kaplan-Meier survival curves of distant metastasis-free survival (DMFS) and overall survival (OS) in relation to nuclear YAP1 expression. Patients with high nuclear YAP1 expression exhibited poor DMFS and overall survival (OS) (A, HR 2.271, 95% CI 1.109–4.650, P = 0.0249; B, HR 3.856, 95%CI 1.321–11.26; P = 0.0135, log-rank test, respectively).
Negative hormone receptor status, tumor size >2 cm, and high nuclear YAP1 expression were significantly associated with decreased DMFS, as assessed by univariate analysis ( Table 2 ). High nuclear YAP1 expression was still a significant determinant of decreased DMFS after adjustment for hormone receptor status, tumor size, and nuclear YAP1 expression by the Cox proportional hazards model (HR 1.893, 95% CIs 1.009–3.552, P = 0.047).
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for distant metastasis-free survival (DMFS).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HRs (95% CIs) | P value | HRs (95% CIs) | P value | |
| Age | 0.983 (0.953–1.014) | 0.284 | ||
| HR | 0.015 | 0.158 | ||
| Negative | Reference | Reference | ||
| Positive | 0.449 (0.236–0.854) | 0.607 (0.303–1.215) | ||
| HER2 | 0.295 | |||
| Negative | Reference | |||
| Positive | 0.575 (0.204–1.619) | |||
| HG | 0.101 | |||
| I, II | Reference | |||
| III | 1.670 (0.904–3.083) | |||
| Tumor size | 0.009 | 0.012 | ||
| ≤2 cm | Reference | Reference | ||
| >2 cm | 2.525 (1.263–5.049) | 2.426 (1.211–4.861) | ||
| Lymph node metastasis | 0.051 | |||
| Negative | Reference | |||
| Positive | 1.840 (0.996–3.397) | |||
| YAP1 expression | 0.028 | 0.047 | ||
| Low | Reference | Reference | ||
| High | 2.020 (1.078–3.784) | 1.893 (1.009–3.552) | ||
| Ki67 (%) | 0.237 | |||
| ≤20% | Reference | |||
| >20% | 1.530 (0.756–3.095) | |||
| Lymphovascular invasion | 0.391 | |||
| Negative | Reference | |||
| Positive | 1.411 (0.643–3.099) | |||
| TILs | 0.988 (0.971–1.006) | 0.200 | ||
HR, hormone receptor; HER-2, human epidermal growth factor receptor-2; HG, histologic grade; TILs, tumor-infiltrating lymphocytes.
In the univariate Cox proportional hazard model, negative hormone receptor status, high histologic grade, tumor size > 2 cm, and high nuclear YAP1 expression were found to be significant prognostic factors for OS ( Supplementary Table 2 ). However, in the multivariate analysis, nuclear YAP1 expression was not a significant predictor of OS ( Supplementary Table 2 , HR 1.576, 95% CIs 0.616–4.034, P = 0.343).
In validation cohort, high nuclear YAP1 expression was significantly predictive of decreased DFS ( Supplementary Figure 1 ; HR, 3.208, 95% CIs 1.313–7.833, P = 0.0105, log rank test). ER negativity, PR negativity, tumor size >2 cm, lymph node metastasis, and high nuclear YAP1 expression were significant factors in the multivariate analysis of DFS ( Supplementary Table 3 ). When adjusted for other factors, high nuclear YAP1 expression was a significant factor in reduced DFS (HR 2.112, 95% CIs 1.083–4.119, P = 0.028).
Prognostic Significance of Nuclear YAP1 Expression in TNBC Patients
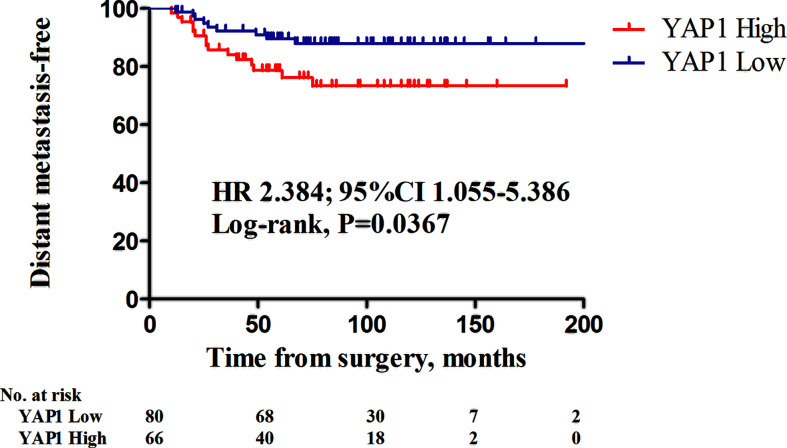
The impact of nuclear YAP1 expression on survival and clinical characteristics was evaluated in patients with TNBC ( Supplementary Table 4 ). Of the 146 TNBC patients, 80 (54.8%) had tumors with low nuclear YAP1 expression, while 66 (45.2%) had tumors with high nuclear YAP1 expression. In TNBC patients, the clinical characteristics were not significantly affected by the level of nuclear YAP1 expression. However, high nuclear YAP1 expression was associated with poor DMFS ( Figure 3 ; HR 2.384, 95% CIs 1.055–5.386, P = 0.0367, log rank test). Lymph node metastasis and high nuclear YAP1 expression were significant determinants of poor DMFS in the univariate analysis ( Supplementary Table 5 ). Adjustment for significant factors in the univariate analysis confirmed that high nuclear YAP1 expression was significantly associated with DMFS (HR 2.329, 95% CIs 1.016–5.339, P = 0.046).
Figure 3.
Kaplan-Meier survival curves of distant metastasis-free survival (DMFS) in relation to nuclear YAP1 expression in triple-negative breast cancers (TNBCs). Patients with high nuclear YAP1 expression exhibited poor DMFS (HR 2.384, 95% CI 1.055–5.386, P = 0.0367; log-rank test).
Discussion
In this study, the nuclear expression of YAP1 was evaluated in a large number of breast cancer specimens, was found to be significantly associated with the occurrence of distant metastasis (HR 2.271, 95%CIs 1.109–4.650, P = 0.0249). Furthermore, nuclear YAP1 expression was a strong determinant of metastasis in TNBC (HR 2.384, 95%CIs 1.055–5.386, P = 0.0367), an aggressive subtype of breast cancer. These findings indicate that targeted therapy for YAP1 may potentially improve the survival outcomes, particularly metastasis, of the patients with breast cancer.
The activation of YAP1, along with that of another transcriptional co-activator TAZ, is associated with dysregulated Hippo signaling (33, 34). YAP1 overexpression promotes EMT, inhibits apoptosis, and induces growth factor-independent cell proliferation. Based on these findings, it was speculated that YAP1 may play a role as a proto-oncogene (23). In addition, several studies have reported a negative impact of YAP1 activation on the survival of patients with gastric, colorectal, ovarian, bladder, and non-small cell lung cancer (22, 29, 35–37).
Currently, the role of YAP1 in breast cancer remains controversial. For instance, Lehn and colleagues reported that YAP1 expression is inversely correlated with HG and tumor cell proliferation, and that low YAP1 mRNA levels are associated with decreased recurrence-free survival and tamoxifen-resistance in luminal A subtype breast cancer (38). Also, some have reported that YAP1 promotes cell proliferation, tumorigenesis, EMT, and drug resistance, and is associated with TP53 mutation, ER negativity, and poor survival (18, 39). However, several other studies have suggested that survival is not significantly affected by YAP1 expression in breast cancer (39, 40), or YAP1 may in fact act as a tumor suppressor (26, 41). Among the most of previous studies mentioned above, the specific argument on subcellular localization of YAP1 in previous studies were unclear. Although Vlug et al. showed upregulated YAP1 expression in invasive lobular carcinoma, but its prognostic impact was not evaluated (39). Kim et al. evaluated the differential expression of YAP and phosphorylated YAP in the each of molecular subtypes of breast cancer and found that nuclear YAP expression was associated with shorter survival (20). However, in previous study, nuclear YAP expression in multivariate analysis did not have statistical significance. Given that nuclear YAP1 expression is a surrogate marker of an activated form of YAP1 (9), we focused on the nuclear YAP1 expression in this study, and found that nuclear YAP1 expression is an independent prognostic predictor for distant metastasis, particularly in the TNBC patients.
TNBC is simply defined as a breast cancer that satisfied the ER, PR, and HER2 negativities, however it is not a uniform subtype rather a complex heterogeneous collection of molecularly different subtypes (42). Unlike hormone receptor-positive type or HER2-type breast cancer, there is currently limited therapeutic targets for TNBC, Although immune checkpoint inhibitors are now applied in TNBC in combination with chemotherapy (43, 44), demanding on targeted therapy is still existed, and YAP1 could be the one of candidate of potential therapeutic target.
Our study has several limitations. First, it was a retrospective study. Second, the TNBC subtype was overrepresented due to selection bias during sample collection. Third, TMA slide staining may underestimate the rate of YAP1 positivity compared to whole-slide examination. Although our study is difficult to compare with previous IHC studies (20, 26, 38, 40, 41, 45), as these did not clarify whether the employed antibodies were specific for phosphorylated YAP1, nor was the intracellular localization of the antigen established, we specifically examined the nuclear YAP1 expression and confirmed its prognostic effect. In our study, no correlations were found between cytosolic YAP1 staining and survival (data not shown). Despite these limitations, the results demonstrated that nuclear YAP1 expression was a clinical prognostic factor in breast cancer, especially TNBC.
In conclusion, we suggested that nuclear YAP1 expression is a clinical prognostic factor for breast cancer. In addition, YAP1 is a potentially valuable therapeutic target for patients with breast cancer, especially in TNBC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The protocol was approved by the institutional review board (Local IRB number: 3-2019-0188) of Gangnam Severance Hospital. The need for informed consent was waived under the approval of the IRB due to the retrospective design.
Author Contributions
YC and CY had full access to all of data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Conceptualization, YC and CY. Data curation, YC, SB, DK, JK, T-KY, W-CP, AL, and CY. Funding acquisition, YC. Investigation, YC, SB, DK, SA, JJ, and CY. Methodology, YC and CY. Resources, JJ and JK. Formal analysis, YC and CY. Supervision, JJ. Writing—original draft, YC and CY. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a faculty research grant from the Yonsei University College of Medicine (6-2018-0080).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a pre-print at Research Square (Yoon Jin Cha et al.).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.609743/full#supplementary-material
References
- 1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin (2007) 57(1):43–66. 10.3322/canjclin.57.1.43 [DOI] [PubMed] [Google Scholar]
- 2. Jurisic V, Radenkovic S, Konjevic G. The Actual Role of LDH as Tumor Marker, Biochemical and Clinical Aspects. Adv Exp Med Biol (2015) 867:115–24. 10.1007/978-94-017-7215-0_8 [DOI] [PubMed] [Google Scholar]
- 3. Radenkovic S, Konjevic G, Gavrilovic D, Stojanovic-Rundic S, Plesinac-Karapandzic V, Stevanovic P, et al. pSTAT3 expression associated with survival and mammographic density of breast cancer patients. Pathol Res Pract (2019) 215(2):366–72. 10.1016/j.prp.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 4. Lotfinejad P, Asghari Jafarabadi M, Abdoli Shadbad M, Kazemi T, Pashazadeh F, Sandoghchian Shotorbani S, et al. Prognostic Role and Clinical Significance of Tumor-Infiltrating Lymphocyte (TIL) and Programmed Death Ligand 1 (PD-L1) Expression in Triple-Negative Breast Cancer (TNBC): A Systematic Review and Meta-Analysis Study. Diagnostics (Basel) (2020) 10(9):704. 10.3390/diagnostics10090704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferris RL, Lotze MT, Leong SP, Hoon DS, Morton DL. Lymphatics, lymph nodes and the immune system: barriers and gateways for cancer spread. Clin Exp Metastasis (2012) 29(7):729–36. 10.1007/s10585-012-9520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer (1989) 63(1):181–7. [DOI] [PubMed] [Google Scholar]
- 7. Atkinson EN, Brown BW, Montague ED. Tumor volume, nodal status, and metastasis in breast cancer in women. J Natl Cancer Inst (1986) 76(2):171–8. 10.1093/jnci/76.2.171 [DOI] [PubMed] [Google Scholar]
- 8. https://seer.cancer.gov/archive/publications/survival/. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988-2001, Patient and Tumor Characteristics.
- 9. Lee CK, Jeong SH, Jang C, Bae H, Kim YH, Park I, et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science (2019) 363(6427):644–9. 10.1126/science.aav0173 [DOI] [PubMed] [Google Scholar]
- 10. Pan D. The hippo signaling pathway in development and cancer. Dev Cell (2010) 19(4):491–505. 10.1016/j.devcel.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev (2016) 30(1):1–17. 10.1101/gad.274027.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res (2009) 69(3):1089–98. 10.1158/0008-5472.Can-08-2997 [DOI] [PubMed] [Google Scholar]
- 13. Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev (2007) 21(21):2747–61. 10.1101/gad.1602907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen J, Cao B, Wang Y, Ma C, Zeng Z, Liu L, et al. Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J Exp Clin Cancer Res (2018) 37(1):175. 10.1186/s13046-018-0850-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng H, Zhang Z, Rodriguez-Barrueco R, Borczuk A, Liu H, Yu J, et al. Functional genomics screen identifies YAP1 as a key determinant to enhance treatment sensitivity in lung cancer cells. Oncotarget (2016) 7(20):28976–88. 10.18632/oncotarget.6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov (2014) 13(1):63–79. 10.1038/nrd4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep (2014) 15(6):642–56. 10.15252/embr.201438638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol (2008) 20(6):638–46. 10.1016/j.ceb.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim DH, Kim SH, Lee OJ, Huang SM, Kwon JL, Kim JM, et al. Differential expression of Yes-associated protein and phosphorylated Yes-associated protein is correlated with expression of Ki-67 and phospho-ERK in colorectal adenocarcinoma. Histol Histopathol (2013) 28(11):1483–90. 10.14670/hh-28.1483 [DOI] [Google Scholar]
- 20. Kim SK, Jung WH, Koo JS. Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer. Int J Clin Exp Pathol (2014) 7(6):3224–34. [PMC free article] [PubMed] [Google Scholar]
- 21. Lee KW, Lee SS, Kim SB, Sohn BH, Lee HS, Jang HJ, et al. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin Cancer Res (2015) 21(2):357–64. 10.1158/1078-0432.Ccr-14-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu JY, Li YH, Lin HX, Liao YJ, Mai SJ, Liu ZW, et al. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer (2013) 13:349. 10.1186/1471-2407-13-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A (2006) 103(33):12405–10. 10.1073/pnas.0605579103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur J Cancer (2012) 48(8):1227–34. 10.1016/j.ejca.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 25. Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer (2009) 115(19):4576–85. 10.1002/cncr.24495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ (2008) 15(11):1752–9. 10.1038/cdd.2008.108 [DOI] [PubMed] [Google Scholar]
- 27. Han SX, Bai E, Jin GH, He CC, Guo XJ, Wang LJ, et al. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res (2014) 2014:261365. 10.1155/2014/261365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fitamant J, Kottakis F, Benhamouche S, Tian HS, Chuvin N, Parachoniak CA, et al. YAP Inhibition Restores Hepatocyte Differentiation in Advanced HCC, Leading to Tumor Regression. Cell Rep (2015) 10(10):1692–707. 10.1016/j.celrep.2015.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci (2010) 101(5):1279–85. 10.1111/j.1349-7006.2010.01511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon CI, Ahn SG, Bae SJ, Shin YJ, Cha C, Park SE, et al. High A20 expression negatively impacts survival in patients with breast cancer. PLoS One (2019) 14(8):e0221721. 10.1371/journal.pone.0221721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol (2010) 28(16):2784–95. 10.1200/jco.2009.25.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol (2018) 36(20):2105–22. 10.1200/jco.2018.77.8738 [DOI] [PubMed] [Google Scholar]
- 33. Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J (2000) 19(24):6778–91. 10.1093/emboj/19.24.6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene (1994) 9(8):2145–52. [PubMed] [Google Scholar]
- 35. Jeong W, Kim SB, Sohn BH, Park YY, Park ES, Kim SC, et al. Activation of YAP1 is associated with poor prognosis and response to taxanes in ovarian cancer. Anticancer Res (2014) 34(2):811–7. [PMC free article] [PubMed] [Google Scholar]
- 36. Song M, Cheong JH, Kim H, Noh SH, Kim H. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res (2012) 32(9):3827–34. [PubMed] [Google Scholar]
- 37. Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A, et al. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One (2013) 8(6):e65539. 10.1371/journal.pone.0065539 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Lehn S, Tobin NP, Sims AH, Stal O, Jirstrom K, Axelson H, et al. Decreased expression of Yes-associated protein is associated with outcome in the luminal A breast cancer subgroup and with an impaired tamoxifen response. BMC Cancer (2014) 14:119. 10.1186/1471-2407-14-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vlug EJ, van de Ven RA, Vermeulen JF, Bult P, van Diest PJ, Derksen PW. Nuclear localization of the transcriptional coactivator YAP is associated with invasive lobular breast cancer. Cell Oncol (Dordr) (2013) 36(5):375–84. 10.1007/s13402-013-0143-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sheen-Chen SM, Huang CY, Tsai CH, Liu YW, Wu SC, Huang CC, et al. Yes-associated protein is not an independent prognostic marker in breast cancer. Anticancer Res (2012) 32(8):3321–5. [PubMed] [Google Scholar]
- 41. Cao L, Sun PL, Yao M, Jia M, Gao H. Expression of YES-associated protein (YAP) and its clinical significance in breast cancer tissues. Hum Pathol (2017) 68:166–74. 10.1016/j.humpath.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 42. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest (2011) 121(7):2750–67. 10.1172/jci45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med (2018) 379(22):2108–21. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 44. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet (2020) 396(10265):1817–28. 10.1016/s0140-6736(20)32531-9 [DOI] [PubMed] [Google Scholar]
- 45. Jaramillo-Rodriguez Y, Cerda-Flores RM, Ruiz-Ramos R, Lopez-Marquez FC, Calderon-Garciduenas AL. YAP expression in normal and neoplastic breast tissue: an immunohistochemical study. Arch Med Res (2014) 45(3):223–8. 10.1016/j.arcmed.2014.01.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.