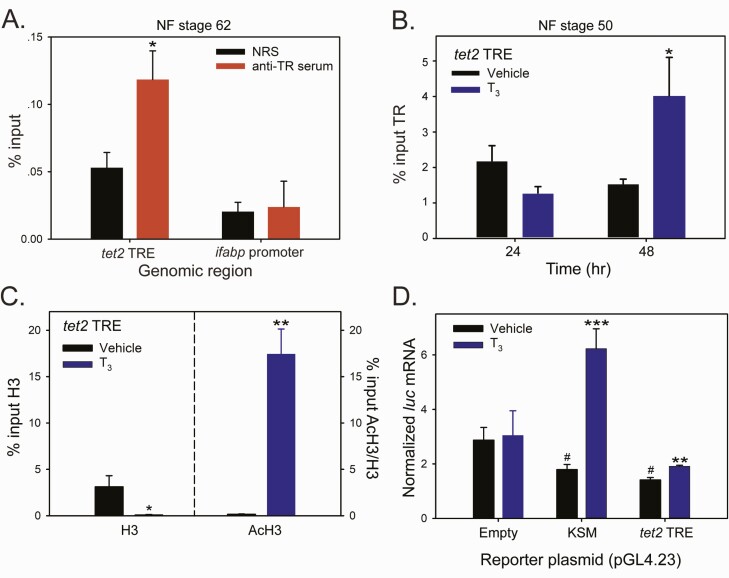
Figure 6.
Thyroid hormone induced chromatin modifications at the putative X. tropicalis tet2 TRE region, and this genomic region supports T3-dependent transactivation. We conducted targeted qPCR-ChIP assays on chromatin isolated from whole brain of metamorphic climax stage (NF stage 62) tadpoles (panel A) or premetamorphic stage (NF stage 50) tadpoles (panel B) treated with vehicle (0.0003% DMSO) or T3 (5 nM) added to their aquarium water for different times. Bars represent the mean ± SEM (n = 4/treatment; experiments were repeated at least twice). Data were analyzed by Student’s independent t-test, and asterisks indicate statistically significant differences between NRS and anti-TR serum (panel A) or vehicle and T3-treated groups (panels B, C, and D; *P < 0.05, **P < 0.01, ***P < 0.001; # in panel D indicates statistically significant differences from vehicle-treated empty vector control; P < 0.0001). A: Targeted qPCR-ChIP assay confirmed TR association in chromatin at the tet2 5’ UTR in metamorphic climax stage tadpole brain. B: Targeted qPCR-ChIP assay showed increased TR ChIP signal at the tet2 5’ UTR in the brain of premetamorphic tadpoles treated with T3 for 48 hours (hr). C: Targeted qPCR-ChIP assays conducted on chromatin isolated from premetamorphic tadpole brain treated with T3 for 12 hours showed that T3 induced nucleosome repositioning at the tet2 5’ UTR, evidenced by a decrease in the H3 ChIP signal, and an open and active chromatin structure, evidenced by a large increase in the AcH3 ChIP signal (normalized to H3). There was no change in H3 or AcH3 at the ifabp promoter region after T3 treatment (data not shown). D: The 5’ UTR region of the X. tropicalis tet2 gene containing putative TREs (see Fig. 4) supports T3-dependent transactivation in transient transfection-reporter assays. We subcloned a 610 bp fragment of tet2 (Supplemental Table 2) (62) into the luciferase reporter vector pGL4.23 and transfected Neuro2a[TRβ1] cells with this vector (pGL4.23-tet2TRE), a vector containing the X. tropicalis klf9 synergy module (pGL4.23-KSM) or empty vector. Twenty hours after transfection, we treated cells with vehicle (0.01% DMSO) or T3 (30 nM) for 12 hours, then harvested for RNA isolation and analysis of luciferase (luc) mRNA by RT-qPCR.