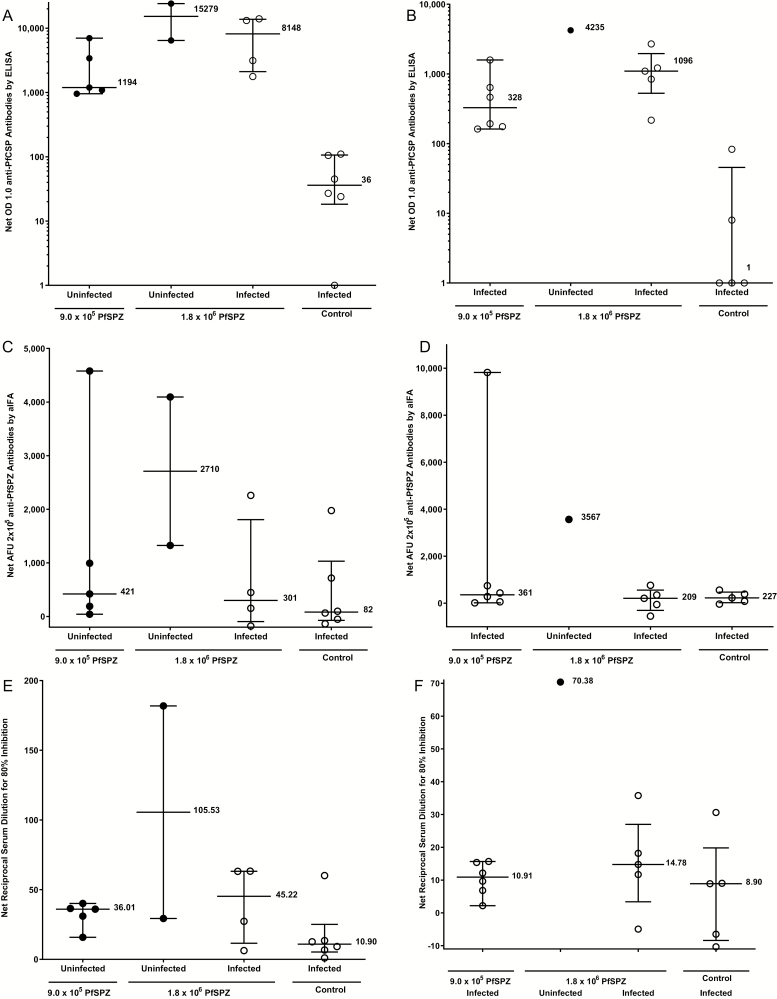
Figure 3.
IgG antibodies to PfCSP by ELISA at time of first CHMI (CHMIs no. 1 and no. 2). A, IgG antibodies to PfSPZ by aIFA (C) and automated inhibition of PfSPZ invasion of hepatoma cells (aISI) (E) and at time of second CHMI (CHMI no.3) by ELISA (B), aIFA (D) and ISI (F) in subjects who received 9 × 105 PfSPZ or 1.8 × 106 PfSPZ doses of PfSPZ Vaccine. Filled circles (●) represent volunteers remaining uninfected after CHMI; open circles (○) represent volunteers infected after CHMI. For the PfCSP ELISA vaccinees were considered to have a positive antibody response if their net OD 1.0 and OD 1.0 ratio, calculated, respectively, by subtracting or dividing the OD 1.0 by the prevaccination OD 1.0, were ≥ 50 and ≥ 3.0. By these criteria, in the 9.0 × 105 PfSPZ group, 5/6, 6/6, and 1/6 were positive 2 weeks after third dose, and before their first and second CHMIs. In the 1.8 × 106 PfSPZ group 6/6, 6/6, and 5/6 were positive 2 weeks after third dose, and before their first and second CHMIs. No control volunteers were positive at any time point. For preimmunization anti-PfCSP levels, there were no significant differences between infected and noninfected vaccinees (Table S6). In the aIFA, volunteers with a net arbitrary fluorescence units (AFU) 2 × 105 of ≥150 and a ratio of post to pre AFU 2 × 105 of ≥ 3.0 were considered positive (Table S5). By these criteria, in the 9.0 × 105 PfSPZ group, 5/6, 5/6, and 4/6 were positive 2 weeks after the third dose, and before their first and second CHMIs (Table S5). In the 1.8 × 106 PfSPZ group, 5/6, 5/6, and 4/6 were positive 2 weeks after the third dose, and before their first and second CHMIs (Table S5). Antibodies to PfSPZ at time of first and second CHMIs by aIFA are shown in Figure 3C and 3DTable S5. At the time of the first CHMI the 5 uninfected (protected) subjects in the 9 × 105 PfSPZ group had a lower median net AFU 2 × 105 (421) than did the 2 uninfected (2710) subjects in the 1.8 × 106 group and higher than the 4 infected (301) subjects in the 1.8 × 106 group, but the differences were not significant (P = .38 and .73). At the time of the second CHMI the 6 infected subjects in the 9 × 105 PfSPZ group had a lower median net AFU 2 × 105 (361) than did the one uninfected (3567) subject in the 1.8 × 106 group and higher than the 5 infected (209) subjects in the 1.8 × 106 group (P = .57 and .43). For the aISI, volunteers with a net ISI reciprocal serum dilution for 80% inhibition of ≥10 and ratio of post to preimmune ISI reciprocal serum dilution for 80% inhibition of ≥ 3.0 were considered positive. By these criteria, in the 9.0 × 105 PfSPZ group, 0/6, 2/6, and 2/6 were positive 2 weeks after the third dose and before their first and second CHMIs (Table S5). In the 1.8 × 106 PfSPZ group, 2/6, 4/6, and 3/6 were positive 2 weeks after the third dose, and before their first and second CHMIs (Table S5). Antibodies to PfSPZ at time of first and second CHMIs by ISI are shown in Figure 3E and 3F and Table S5. At time of the first CHMI the 5 uninfected (protected) subjects in the 9 × 105 PfSPZ group had a nonsignificant lower median net reciprocal serum dilution for 80% inhibition (22.57) than did the 2 uninfected (95.19) and 4 infected (34.12) subjects in the 1.8 × 106 group, but the differences did not reach the level of statistical significance (P = .095 and .90). At the time of the second CHMI the 6 infected subjects in the 9 × 105 PfSPZ group had a nonsignificant lower median net reciprocal serum dilution for 80% inhibition (10.91) than did the one uninfected (70.38) subject in the 1.8 × 106 group and the 5 infected (14.78) subjects in the 1.8 × 106 group (P = .29 and .54). Abbreviations: aIFA, automated immunofluorescence assay; CHMI, controlled human malaria infection; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; ISI, inhibition of sporozoite invasion assay; PfCSP, Pf circumsporozoite protein; PfSPZ, Plasmodium falciparum sporozoite.