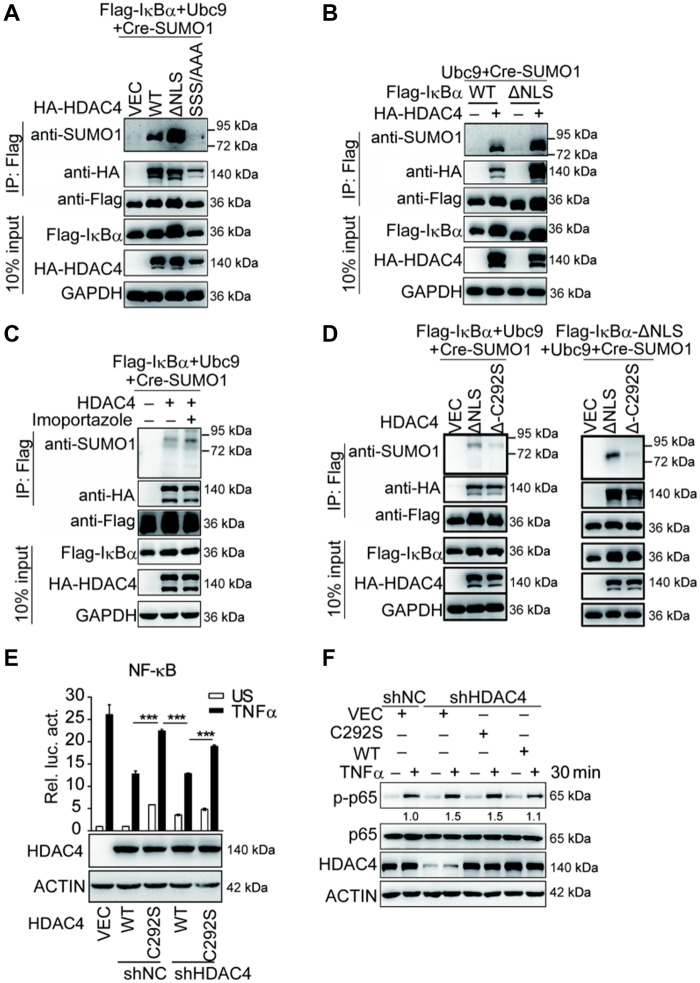
Figure 7.
The contribution of HDAC4 C292 to the activation of NF-κB signaling. (A) The sumoylation of IκBα requires cytoplasmic localization of HDAC4. HEK293T cells (1 × 107) were cotransfected with Cre-SUMO1, Ubc9, Flag-IκB, and the indicated expression plasmids for WT HDAC4, HDAC4-ΔNLS, or HDAC4-SSS/AAA. Sumoylation assays and immunoblotting analysis were performed 36 h after transfection. (B) Sumoylation of IκBα-ΔNLS or IκBα-ΔNES. The experiments were performed as in A except that the indicated plasmids were used. (C) The inhibition of nuclear localization of HDAC4 enhances the sumoylation of IκBα. The HEK293T cells (1 × 107) were transfected with the indicated expression plasmids for 36 h and then treated with imoportazole (20 nM). The sumoylation assays and immunoblotting analysis were performed as in A. (D) Effect of HDAC4-ΔNLS-C292S on the sumoylation of IκBα in overexpression sumoylation assays. The experiments were performed as in A except that the indicated plasmids were used. (E) The enzyme-inactive mutant HDAC4 (C292S) potentiates the promoter activity of NF-κB in HDAC4-stable knockdown HEK293T cells. The control cells (shNC) or HDAC4-stable knockdown HEK293T cells (shHDAC4) reconstituted with WT HDAC4 or HDAC4 C292S mutant were transfected with the NF-κB luciferase reporter plasmid (0.01 µg). Twenty-four hours after transfection, cells were treated with TNFα (20 ng/ml) or left untreated for 10 h before luciferase assays were performed. (F) HDAC4 C292S enhances the phosphorylation of NF-κB compared with WT HDAC4 in HDAC4-stable knockdown HEK293T cells. The experiments were performed as in E, except that the indicated cells were induced with TNFα (20 ng/ml) for 30 min before the immunoblotting analysis. Data are representative of three independent experiments. Graphs show mean ± SD; n = 3. ***P < 0.001.