Figure 1.
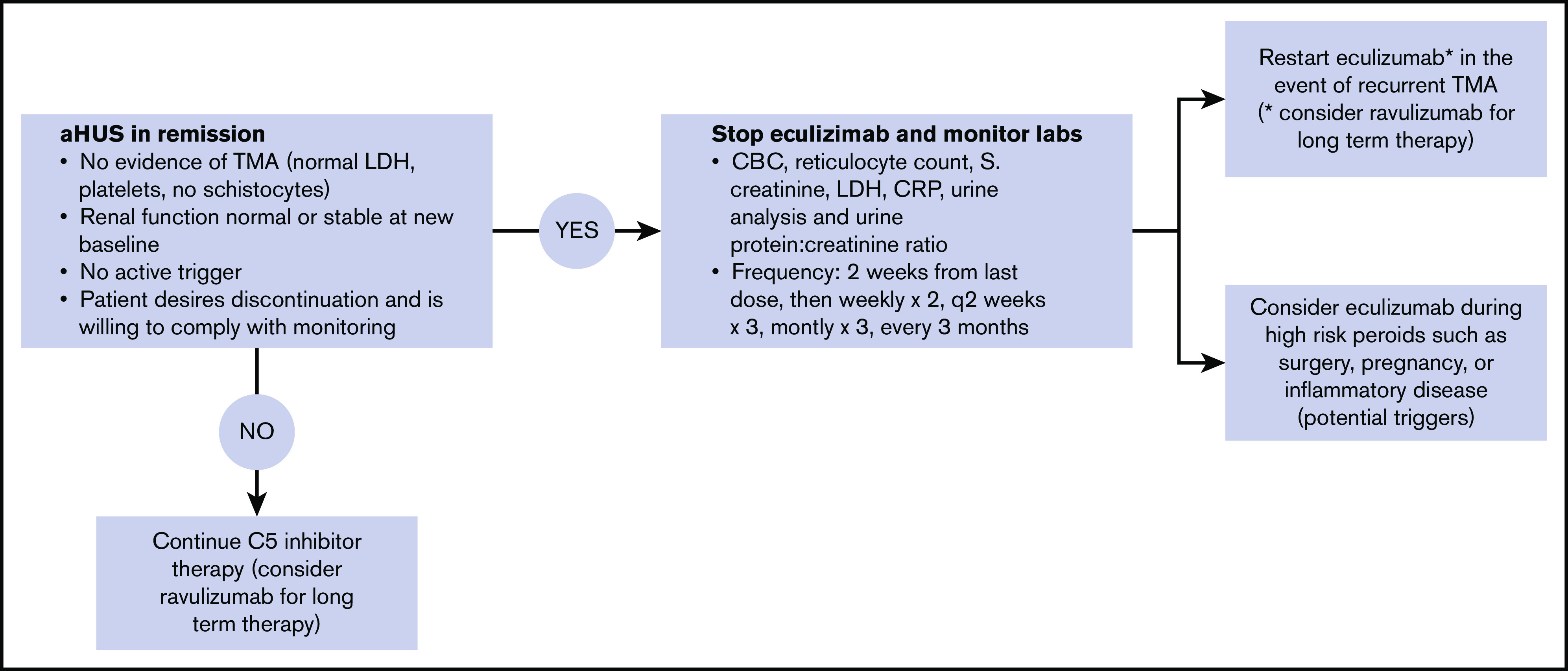
Protocol for eculizumab discontinuation and monitoring. All 4 of the following criteria must be met before we discontinue eculizumab: resolved TMA, renal function normal or stable at new baseline, no active trigger (in patients that had an identified trigger), and patients desire to stop therapy and agree to monitoring plan. Monitoring is conduced as outlined. Home urine dipstick monitoring may also be used as an adjunct. We restart a C5 inhibitor (eculizumab or ravulizumab) in the case of recurrent TMA, in which case therapy is continued indefinitely or possibly temporarily during high-risk periods such as pregnancy, surgery, or flare of inflammatory disease. When long-term therapy is anticipated, we suggest ravulizumab rather than eculizumab.
