Abstract
Introduction
The coronavirus disease‐19 (COVID‐19) pandemic presents challenges to the conduct of randomized clinical trials of lifestyle interventions.
Methods
World‐Wide FINGERS is an international network of clinical trials to assess the impact of multidomain lifestyle intervention on cognitive decline in at‐risk adults. Individual trials are tailoring successful approaches from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) to local cultures and environments. The network convened a forum for researchers to discuss statistical design and analysis issues they faced during the pandemic. We report on experiences of three trials that, at various stages of conduct, altered designs and analysis plans to navigate these issues. We provide recommendations for future trials to consider as they develop and launch behavioral intervention trials.
Results
The pandemic led researchers to change recruitment plans, interrupt timelines for assessments and intervention delivery, and move to remote intervention and assessment protocols. The necessity of these changes add emphasis to the importance, in study design and analysis, of intention to treat approaches, flexibility, within‐site stratification, interim power projections, and sensitivity analyses.
Discussion
Robust approaches to study design and analysis are critical to negotiate issues related to the intervention. The world‐wide network of similarly oriented clinical trials will allow us to evaluate the effectiveness of responses to the pandemic across cultures, local environments, and phases of the pandemic.
Keywords: cognitive function, COVID‐19 pandemic design, lifestyle interventions, randomized controlled clinical trials
1. INTRODUCTION
The coronavirus disease‐19 (COVID‐19) pandemic has taken a staggering toll on public health. As of mid‐November 2020, more than 54 millions cases and almost 1.2 million deaths have been reported world‐wide (https://coronavirus.jhu.edu). In attempting to slow rates of infection, countries have imposed travel restrictions, physical distancing, and rigorous infection control measures at health‐care institutions. 1 These measures pose important consequences for the conduct of randomized clinical trials.
Many of these consequences have necessitated changes and adjustments in study protocols, to accommodate suspension of in‐person data collection, alterations in approaches to recruitment, and changes in intervention delivery. 2 , 3 , 4 Common concerns expressed by study teams are barriers to participant enrollment, health concerns of study staff, and financial concerns related to study suspensions and cancellations. 5 Further, in some studies ethical concerns have been raised regarding the conduct of trials related to participant safety and the potential that interventions may adversely interact with COVID‐19 infection. 6 Even trial monitoring is affected, with the imperative of streamlining how protocols are evaluated and safety data are collected. 7
Lifestyle intervention trials may be particularly susceptible to disruption from the pandemic. Traditionally, many of these have focused on developing strong bonds between interventionists and participants through group and individual face‐to‐face sessions, and past work has highlighted that these interactions are important for sustaining high levels of adherence, retention, and well‐being. Often, they are delivered in the community. Pandemic control measures may present additional challenges to maintain participant safety and to comply with local regulations. Lifestyle behaviors may be adversely affected by social isolation imposed by self‐distancing, in part due to challenges related to the comorbidities of increased anxiety and depression. 8 , 9 , 10 Trials focused on interventions to prevent cognitive decline often recruit individuals for whom COVID‐19 poses greater risks due to their older age and greater burden of age‐related chronic diseases.
The focus we bring to the discussion is the impact of the pandemic on the statistical design and analysis plans for ongoing clinical trials. This article highlights experience from the World‐Wide FINGERS (WW‐FINGERS), a network of clinical trials to assess the relative impact of multidomain lifestyle intervention on cognitive function, 11 and the discussion below focuses on how such trials face challenges in different environments and different stages in the trial, that is, during recruitment, intervention delivery, and longer term follow‐up. The WW‐FINGERS network provides a forum for studies to share and learn from one another. In particular, the global response and different regional responses have enriched the context of this sharing and conversation throughout 2020. How ongoing studies have responded to local environments, making adjustments to protocols at different stages of trial conduct, informs our discussion.
2. THE WW‐FINGERS NETWORK
WW‐FINGERS is the first network of multidomain lifestyle‐based intervention trials for risk reduction and prevention of cognitive impairment and dementia in older adults. 11 WW‐FINGERS builds upon the successful experience of FINGER: the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (ClinicalTrials.gov NCT01041989), a randomized controlled trial (RCT) that showed the feasibility and efficacy of a multimodal lifestyle intervention consisting of nutritional guidance, exercise, cognitive training, and control of vascular and metabolic risk factors. FINGER enrolled 1260 community dwellers in Finland who were aged 60 to 77 and at increased risk of dementia, and who were randomized 1:1 to the multidomain intervention or a group receiving regular health advice. 12 , 13 The 2‐year lifestyle intervention, compared to general health advice, resulted in improved global cognition, the primary outcome of the study. It also showed positive effects in several secondary outcomes, including cognitive subdomains, mobility and functional status, development of chronic disease, and health‐related quality of life. 14 , 15 , 16 , 17 Extended follow‐up of study participants is ongoing to monitor long‐term effects of the intervention: 5‐ and 7‐year follow‐up assessments have been completed in 2016 and 2018, respectively, and a 10‐year follow‐up assessment was planned for 2020, but has been postponed to 2021 due to the COVID‐19 pandemic.
The positive results of the FINGER RCT prompted the need to further test, adapt, and optimize the FINGER model in other populations and settings, leading to the launch in 2017 of the WW‐FINGERS network, which has been rapidly expanding to currently include approximately 30 countries around the world. 11 , 18
WW‐FINGERS comprises studies at different stages of implementation and with varying levels of alignment to the original FINGER trial. 11 Key methodological features common to the trials include assessment of a multidomain intervention aiming to ameliorate vascular, metabolic, and lifestyle‐related factors associated with increased risk of cognitive decline and dementia; delivery of the intervention using both individual and group sessions, to optimize the intervention at an individual level, but also to provide social stimulation; use of randomization to ensure proper comparison among the trials´ arms; and prospective harmonization of cognitive outcomes (i.e., assessment of cognitive changes) and other outcomes (e.g., functional status). 11 Importantly, the WW‐FINGERS network does not aim to replicate the FINGER intervention in its original form, as adaptation of the FINGER multidomain intervention model is deemed crucial for its successful implementation in different cultural settings and populations, and ultimately to define sustainable prevention strategies that are optimized for different at‐risk groups. Toward this aim, the network research teams collaborate to develop intervention protocols that are appropriate for different populations in various geographical and cultural settings, taking into account local factors (e.g., country‐specific food habits and availability; national guidelines for clinical management of hypertension, diabetes, and dyslipidemia). Research teams are able to adopt advances in the delivery of individual domains of the intervention, for example, increasing the intensity of the physical activity component, revising nutritional goals, and adding emphasis to promoting vascular health. In addition, the network promotes harmonization of outcomes and measurements, aiming to allow for combined data analyses evaluating, for example, subgroup analyses. Network activities are aligned with the World Health Organization (WHO) Global action plan on the public health response to dementia 2017–2025, the WHO guidelines for risk reduction of cognitive decline and dementia, and recommendations from the latest report of the Lancet Commission on dementia prevention, intervention, and care. 19 , 20 , 21
RESEARCH IN CONTEXT
Systematic Review: The World‐Wide FINGERS network developed a forum for participating trialists to discuss challenges and approaches to conducting clinical trials of multidomain lifestyle interventions during the coronavirus disease‐19 (COVID‐19) pandemic.
Interpretation: Study groups have responded to the pandemic by altering assessment schedules and intervention delivery, which has raised issues for statistical design and analysis plans. We summarize these and provide recommendations for how future trials should navigate these issues.
Future Directions: The World‐Wide FINGERS network is continuing to develop well‐designed clinical trials to identify effective strategies to slow cognitive decline and potentially lower risks for Alzheimer's disease and dementia.
The COVID‐19 pandemic has affected all WW‐FINGERS countries, disrupting activities for many studies that are in different stages: from study initiation, to participant recruitment, intervention delivery and monitoring, and post‐intervention extended follow‐up. The network has started a series of virtual meetings to discuss the different methodological challenges related to the pandemic and propose ways forward.
In the following sections, three ongoing WW‐FINGERS RCTs are presented as examples of methodological challenges on lifestyle‐based interventions in older adults aiming to reduce the risk of dementia. The trials have been affected by the pandemic: (1) mainly in the recruitment process (J‐MINT: Japan‐Multimodal Intervention Trial for Prevention of Dementia), (2) during recruitment and intervention delivery (U.S. POINTER: U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk), and (3) during intervention adherence and post‐intervention follow‐up (German AgeWell.de study; see Figure 1). For each RCT, we summarize main aspects of the study design and the major statistical and design issues associated with the pandemic interference. We then discuss lessons learned and a way forward to address methodological challenges and identify new opportunities.
FIGURE 1.
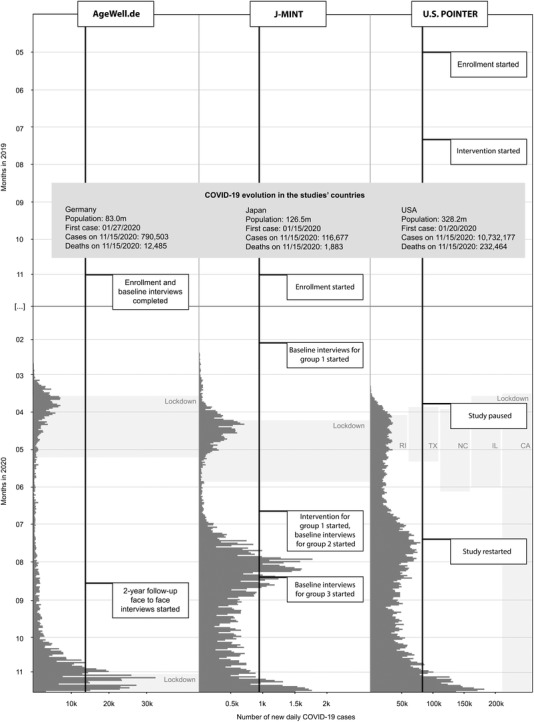
Timeline of the WW‐FINGERS studies AgeWell.de, J‐MINT, and U.S. POINTER and the evolution of COVID‐19 cases in the countries of study conduction, Germany, Japan, and the United States, respectively. Abbreviations: CA, California; COVID‐19, coronavirus disease‐19; IL, Illinois; J‐MINT, Japan‐Multimodal Intervention Trial for Prevention of Dementia; NC, North Carolina; RI, Rhode Island; TX, Texas; U.S. POINTER, U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk; WW‐FINGERS, World‐Wide Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability
3. J‐MINT
3.1. Study design
The J‐MINT RCT is a multicenter RCT of an 18‐month multidomain intervention for dementia prevention, in which participants are adults with mild cognitive impairment, aged 65 to 85 years. It is organized by the National Center for Geriatrics and Gerontology (NCGG) as a central coordinating center, and involves four other centers located in Japan: Nagoya University, Nagoya City University, Fujita Health University, and Tokyo Metropolitan Institute of Gerontology. J‐MINT plans to recruit 500 participants and randomly assign them into intervention and control groups. The intervention includes four domains: management of vascular risk factors (diabetes, hypertension, obesity, and dyslipidemia), group‐based physical exercise program and increased physical activity, nutritional counselling, and cognitive training using BrainHQ (© 2020 Posit Science). Control group participants are sent health‐related information every 2 months. Cognitive function and other measures including biomarkers and brain imaging are assessed at baseline, 6, 12, and 18 months in both groups. J‐MINT enrollment began in November 2019.
3.2. Impact of COVID‐19 on J‐MINT
Japan had sporadic clusters of COVID‐19 through visitors and young travelers returning from abroad until late March 2020 (Figure 1). To prevent outbursts of the infection, the Japanese government declared a state of emergency in seven large cities, including Tokyo and Osaka, on April 7, and it declared a national state of emergency for a month beginning on April 16. This was lifted in late May when infection rates declined. The government recommended physical distancing, avoiding places where the “3Cs” (closed spaces, crowded places, and close‐contact settings) overlap, hand hygiene, and wearing a face mask. In spite of these measures, Japan observed the second wave of the COVID‐19 outbreak from July as shown in Figure 1.
Due to the COVID‐19 pandemic, the J‐MINT study has been forced to halt the recruitment and initial evaluation of participants from late February to mid‐May 2020. Enrollment activities resumed in late May from NCGG, with appropriate measures against infection based on the recommendation of the infection control team of NCGG (body temperature check, hand hygiene, wearing a face mask, ventilation of the room, and physical distancing). The number of people recruited per day decreased, because more space and time were needed for neuropsychological and other tests than before the outbreak. In addition, participants who provided the consent for study participation significantly decreased due to fear of COVID‐19. Compared to the schedule originally planned, the progress of the study has been delayed more than half a year. The number of registered participants was 470 as of October 27, 2020, more than 95% of planned recruitment has been completed and recruitment of new participants is planned to continue until December 2020 according to the protocol.
Interventions were begun in June 2020 with the first groups as NCGG and Tokyo Metropolitan Institute of Gerontology in Tokyo, where the highest number of confirmed COVID‐19 cases were seen in Japan. J‐MINT is increasing the number of interventions in other sites as well. In the physical exercise component of the intervention, instructors wear a mask and a face shield for infection control and participants wear a mask or a mouth shield with appropriate physical distancing (Figure 2). J‐MINT is preparing to provide the intervention of the physical exercise by online delivery in response to the possibility that a third wave of COVID‐19 might come. Infection control measures were a heavy burden for the staff and added to costs.
FIGURE 2.

A J‐MINT instructor wears a face mask and a face shield for infection control, while participants wear a mask or a face shield. Instructors and participants are exercising with physical distancing. J‐MINT, Japan‐Multimodal Intervention Trial for Prevention of Dementia
3.3. Design and analytical consequences
COVID‐19 had a significant impact on the study design of J‐MINT and caused a potential statistical problem. The intervention was originally planned to start in April 2020, but was delayed for 2 months. Therefore, there is no effect of the first waves of the pandemic on the intervention, per se. However, there was a gap of 4 months on average from the initial evaluation to the intervention. The main statistical issue is how to deal with the 4‐month gap period. Currently, J‐MINT plans to examine the impact of the time between the initial evaluation to see if this is correlated with the magnitude of cognitive changes. To this end, J‐MINT investigators will compare the changes in the primary outcome, the composite scores of neuropsychological tests in the control and intervention groups, after adjusting for the time between the initial evaluation and the start of the intervention as a covariate. If the time between the initial evaluation and the start of the intervention is longer than 6 months, the team will conduct a reevaluation and may conduct a subanalysis by excluding the participants who were reevaluated after comparing the results of the initial evaluation with those of the reevaluation.
4. U.S. POINTER
4.1. Study design
U.S. POINTER is a two‐arm, five‐site (Illinois, Texas, North Carolina, Northern California, and Rhode Island) RCT designed to assess the relative impact of two multidomain lifestyle interventions (self‐guided vs. structured) on cognitive function across 2 years of planned follow‐up. The interventions, modeled after the FINGER trial, set goals for increased physical activity, improved diet, cognitive and social stimulation, and risk factor monitoring. At baseline, participants are 60 to 79 years old, sedentary, do not already consume a healthy diet, and are at an increased risk for cognitive decline. Enrollment began in May 2019, at the North Carolina site and the first intervention sessions were initiated 2 months later. Enrollment at the Northern California site began in the fall of 2019.
4.2. Impact of COVID‐19 on study
The first COVID‐19 case in North Carolina was announced in early March 2020. Later that month, the state governor released a “stay‐at‐home” order, urging citizens to avoid non‐essential travel. This was lifted in early May and travel restrictions were loosened. Subsequent relaxing of policies occurred in the following months, which maintained recommendations for physical distancing and limited the size of gatherings. The first COVID‐19 case in the Sacramento region of California occurred in late February 2020, followed by a similar pattern of restrictions on travel and gathering, which were subsequently relaxed.
U.S. POINTER imposed a study‐wide pause on in‐person clinic visits and face‐to‐face intervention delivery in March 2020. At the time, 240 participants had enrolled, primarily at the North Carolina and Northern California sites, and 143 had begun intervention sessions (all at the North Carolina site). During the pause, participant contact was maintained through telephone calls and participants were encouraged to continue to meet intervention goals for physical activity, diet, and cognitive stimulation. Clinic visits for enrollment and data collection were restarted in July 2020, with intervention delivery shifting from in‐person meetings to use of video conferencing technology.
4.3. Design and analytical consequences
The 4‐month pause in face‐to‐face contact with U.S. POINTER participants interrupted the collection of outcome data, which was originally scheduled to occur at 6‐month intervals after “intervention initiation” when intervention groups (consisting of approximately 10–15 members assigned to the same arm) held their first “team meeting.” While some data were accumulated continuously during the pause (e.g., safety, health‐care use, objective measures of physical activity, and cognitive training for the multidomain intervention group), others (cognitive and physical function, risk factors) were postponed relative to the timing of planned assessments and rescheduled to resume at the end of the pause.
During the pause, adherence to intervention goals continued to be monitored and participants received encouragement toward meeting these goals even though some activities (e.g., group meeting attendance) were suspended. After the pause, intervention meetings were held remotely and full adherence monitoring was resumed.
The major statistical and design considerations arising from the pandemic‐related changes in procedures relate to alterations in how interventions were delivered and assessment schedules, and the potential that outcomes and adherence may be differentially affected depending on the temporal influences of the pandemic.
5. AGEWELL.DE
5.1. Study design
AgeWell.de is a pragmatic cluster‐randomized, controlled lifestyle trial against cognitive decline, conducted in Germany across five study sites. Guided by the FINGER model, the lifestyle intervention comprises nutritional counseling, physical activity enhancement, cognitive training, and monitoring of vascular risk factors. Expanding on the FINGER model, AgeWell.de addresses additional dementia risk factors: social inactivity and potentially inappropriate medication. Further, interventions for bereavement, grief, and depressive symptoms are provided, if applicable. The individually tailored intervention is administered during a face‐to‐face session by trained study personnel at the participants’ homes. The effectiveness and cost‐effectiveness of the intervention are evaluated against general health advice/treatment, with global cognitive function being the primary outcome measure.
Between June 2018 and October 2019, 1029 dementia‐free community‐dwelling primary care patients aged 60 to 77 years and with a Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) score of ≥9 points were enrolled by general practitioners and assessed at baseline. Face‐to‐face assessments were scheduled at baseline/pre‐intervention and 2‐year follow‐up/post‐intervention. Detailed information on the study design is provided in the study protocol. 22 To date, AgeWell.de has commenced 2‐year follow‐ups, which will be completed in November 2021.
5.2. Impact of COVID‐19 on study
In Germany, the first COVID‐19 case was reported January 28, 2020, followed by a rapid increase of infections. Nationwide infection control measures became effective on March 21 and the first lockdown was enforced until late May. Physical distancing measures remain in place to date. Since September, a resurgence of infections was observed, which prompted a second lockdown. By mid‐November, 790,503 COVID‐19 cases and 12,485 associated deaths were documented. 23
While recruitment and baseline assessments were completed before the pandemic, two challenges to trial conduct arose. First, infection control measures, including the first nationwide lockdown, largely coincided with the study's intervention period, posing the question of how this may interfere with participants’ intervention performance and adherence. As the quarantine measures put restrictions on lifestyles, concerns specifically arose with regard to social and physical activity and mental well‐being. Second, with the pandemic here to stay for the foreseeable future and its undulating course, 2‐year follow‐up face‐to‐face assessments, which commenced in August 2020, are at risk. A change of assessment mode avoiding face‐to‐face would violate the data integrity with respect to establishing trial outcomes compared to baseline assessments. If further lockdowns occur, future assessments may have to be paused.
5.3. Design and analytical consequences
Several measures have been taken to respond to the COVID‐19 impact on AgeWell.de. A mailed survey measuring the impact of the COVID‐19 pandemic on everyday life, social and mental health, and resilience was carried out during the first lockdown among study participants. The intervention group was asked to evaluate the impact on carrying out the intervention. Simultaneously, a representative survey was conducted in the German old‐age population that used identical measures as the AgeWell.de survey. 24 As such, the representative survey provides a reference point for the AgeWell.de population, which may be affected differently due to the presence of risk factors for dementia that also increase vulnerability for severe courses of COVID‐19. Compromised mental and social well‐being during lockdown may impact cognitive function, and as such, jeopardize trial outcomes. AgeWell.de plans to repeat the surveys to gain a longitudinal perspective of the COVID‐19 pandemic impact. Another approach is to investigate intervention adherence synchronously to the pandemic timeline by analyzing the intervention group's study diaries to track whether and how frequent interventions (e.g., exercise, social activities) were carried out on a weekly basis. This information could be used to derive patterns for each interventional component to reveal potential fluctuations associated with the pandemic timeline. However, it is less clear how to account for the impact—if any—in the trial outcome analysis. The magnitude of the impact has to be established. Certainly, subgroup analyses should be conducted to inspect how intervention effects may vary among components.
A safety and hygiene protocol has been developed to ensure the highest possible infection protection for study participants and personnel engaged in 2‐year follow‐up face‐to‐face assessments. Participants are now provided the option to be assessed at study sites, if they do not want visits to their homes. The safety and hygiene protocol adheres to the up‐to‐date recommendations of the Robert Koch Institute, the German public health institute responsible for disease control. Therefore, face‐to‐face assessments were feasible as long as infection levels did not require strict infection control measures, such as further lockdowns. Lockdowns during follow‐up may introduce regional differences and cluster effects between study sites as well as individual variance in follow‐up duration. In fact, a second nationwide lockdown imposed from early November, expected to last a month, led to pausing face‐to‐face assessments. Varying follow‐up duration is a minor issue that will be handled by statistical modeling. Cluster effects may be more difficult in that they may reduce statistical power: these need to be thoroughly investigated by assessing potential biases and intra‐cluster correlations. If study participants are not willing to participate in face‐to‐face assessments, they are offered a telephone interview that will allow for comprehensive nonresponse analyses.
6. LESSONS LEARNED
6.1. Study design
It is unclear the extent that the COVID‐19 pandemic and the associated public health measures to curb the spread of the virus impact participants’ behavior and everyday routines. As long as the pandemic persists, adaptations to accommodate extra safety precautions must be taken in protocols of lifestyle RCTs. It may be important to add measures, for example, to assess well‐being, mental and social health, virus exposure, health‐care access, and intervention adherence/motivation. This may further our understanding of how the COVID‐19 pandemic has impacted trial results. WW‐FINGERS is currently developing a COVID‐19 survey to identify special challenges to inform how interventions might best be tailored to address these issues.
Our collective experience in WW‐FINGERS emphasizes how important it is that study designs be flexible to allow lifestyle intervention components to be delivered in a physically distanced environment, for example promoting social engagement via digital tools and facilitating home‐based physical exercise. Researchers should consider implementing intervention components to address well‐being, mental health, and stress reduction and skills training for coping and resilience that could help participants manage challenging and uncertain times.
Ongoing studies could adopt protocols to increase engagement with participants to bolster continued motivation and adherence and to understand participant concerns. At this point, it is unknown whether remote intervention delivery is as effective as face‐to‐face delivery. It may work well for some intervention components, such as cognitive training, but may be less effective for others, such as physical activity. Additionally, individuals living in challenging socio‐economic environments may face greater barriers for adherence. Findings from a previous multidomain RCT based on a coach‐supported, interactive, internet‐based platform for reduction of vascular and metabolic risk factors in older adults (HATICE: Healthy Ageing Through Internet Counselling in the Elderly, ISRCTN registry 48151589) provide some insights on the feasibility and efficacy of digitally delivered interventions. 25 HATICE data support the feasibility of a multidomain model delivered remotely, although recruitment to this type of intervention can be selective, and factors such as older age and lower education can be barriers to adherence. 26
6.2. Data collection/assessments
Prospective studies may consider alternatives to face‐to‐face assessments, such as computerized and/or remote (telephone, video) assessments. Studies with in‐person assessments will need to adopt safety and hygiene protocols that allow for continued face‐to‐face assessments to avoid a change in assessment modes: consistency is key for pre–post comparisons. Without this consistency, bias may be introduced in trial outcomes. The need for consistency, however, must be balanced against risk of infection: safety is the greatest priority, and safety and hygiene protocols need to be up to‐date.
6.3. Intention‐to‐treat
The impact of the pandemic on the conduct of trials adds emphasis to the importance of study hypotheses being independent of post‐intervention events. Hypotheses based on intention‐to‐treat remain as initially framed, that is, to compare cohorts by random assignment to different intervention programs. While the intervention goals have remained unchanged, protocols for intervention delivery have changed. Particularly for trials of behavioral interventions, it is often the case that the details behind intervention delivery may change over time. This may result from accrued experience in finding ways to optimize adherence or from changes in the nature of the study cohort as it is recruited over time. Examples include the Lifestyle Interventions for Independence for Elders, which revised intervention protocols to include guidelines for the temporary suspensions of its physical activity interventions to address hospitalizations that occurred at high rates in its older cohort, 27 and the Action for Health in Diabetes trial, which mid‐trial ceased providing weight loss medications to individuals assigned to its behavioral intervention who were unsuccessful in losing weight when the drugs were found to be ineffective. 28 While the influence of the COVID‐19 pandemic may be more disruptive than these examples, the importance of adhering to an intention‐to‐treat protocol remains consistent and important for considerations in data analysis plans.
6.4. Stratification by site
Randomization stratification by site is designed to ensure balanced intervention assignment and avoid confounding attributable to local differences in cohorts and environments. 29 As noted above, the dynamic influence of the pandemic on trial operations varied by site and country. Stratification promotes fair comparisons within each site. An advantage of multi‐site trials and networks is that differences in the disruption of trial operations and possibly outcomes among sites provides additional opportunity to study and contrast how environments may influence findings.
6.5. Post hoc power projections
While unknown, it is possible that the COVID‐19 pandemic may adversely impact adherence, attenuate the effect sizes of trial interventions, and increase attrition. It may also be that alterations in the assessment of study outcomes, for example transitioning from clinic‐based to telephone‐based assessment, may increase variance. Each of these may lower statistical power. Study groups may consider altering designs (e.g., increasing enrollment or extending follow‐up) in response. Other trials have navigated issues associated with changes in how outcomes are assessed. 30 , 31 , 32 It is important that any decisions on protocol changes be made by experts who are masked to outcome data by arm to preserve type 1 error.
6.6. Analytical issues
If interventions are altered during the course of trial enrollment, post hoc stratification may be considered, in which participants enrolled prior to the alteration constitute one strata and those enrolled later constituting a second strata. This permits a formal comparison of relative intervention effects as interventions change and may be considered a sensitivity analysis.
Alterations in assessment schedules and differences in assessment time frames among participants pose thorny analytical issues. These confound assessments with secular trends in health and behavior associated with the pandemic. Sensitivity analyses may be required to gauge the potential impact of these factors on results from planned analyses.
Prior to unmasking the study, statistical analysis plans should be reviewed and updated to include all planned modifications and additional analysis to assess the impact of the pandemic disruption on the efficacy analyses.
7. SUMMARY
The COVID‐19 pandemic has profoundly altered the landscape for the design and conduct of RCTs of multidomain lifestyle interventions. Older individuals with comorbid health conditions, many of whom are ideal candidates for WW‐FINGERS interventions, have the greatest health risks. The WW‐FINGERS was originally initiated to support adaptation of a multidomain intervention model to prevent cognitive decline and to harmonize study protocols. The existing network with ongoing studies at different phases now also presents opportunities to evaluate approaches to intervention and data collection that may be more feasible in the context of a pandemic. It has gathered international teams with expertise to address, almost in real time, the methodological challenges that cut across its linked RCTs. It provides an opportunity to synergize and collaborate on solutions that support the overall aims of the network, including successful conduct of each RCT and a way forward to data sharing and joint analyses. This allows studies at earlier stages in their implementation to adjust and pivot to address these potential challenges in study design and analytics, while others that are more advanced will be able to leverage other sites’ expertise to develop and adjust statistical plans to most appropriately manage their specific circumstances. The lessons from the WW‐FINGERS to adapt and cope with the challenges due to COVID‐19 might be helpful also for other existing and planned lifestyle intervention studies.
FUNDING INFORMATION
AgeWell.de: The study “AgeWell.de – a multi‐centric cluster‐randomized controlled prevention trial in primary care” is funded by the German Federal Ministry for Education and Research (BMBF; grants: 01GL1704A, 01GL1704B, 01GL1704C, 01GL1704D, 01GL1704E, 01GL1704F). J‐MINT: Japan Agency for Medical Research and Development (AMED) (20de0107002h0002) FINGERS: Work on this article was supported by the Academy of Finland project grants 287490 and 317465, and related funding from special COVID‐19 call, Joint Program of Neurodegenerative Disorders ‐ EURO‐FINGERS multimodal precision prevention toolbox for dementia in Alzheimer's disease ‐ grant through the following funding organizations under the aegis of JPND—www.jpnd.eu: Finland, Suomen Akatemia (Academy of Finland, 334804); Sweden, Vetenskapsrådet (VR) (Swedish Research Council, 2019‐02226); Alzheimer's Research and Prevention Foundation (US); Knut and Alice Wallenberg Foundation Sweden; Stiftelsen Stockholms sjukhem Sweden; Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse Sweden; Swedish Research Council; Läkarsällskapet COVID‐19 grant; Alzheimerfonden Sweden; Region Stockholm (ALF and NSV grants); Finnish Ministry of Education and Culture; Swedish Research Council for Health, Working Life and Welfare (FORTE). U.S. POINTER: This work was supported by a grant from the Alzheimer's Association (U.S. POINTER‐19‐611541). Additional support was provided by the Wake Forest Alzheimer's Disease Core Center (P30AG049638‐01A1).
CONFLICTS OF INTEREST
There are no conflicts to report beyond listed funding for World‐Wide FINGERS studies.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
The authors thank Emily S. A. Meyers (Alzheimer's Association) and Kristal Morales Perez (Karolinska Institute) for their exceptional work in coordinating the development of this proposal. We also thank the World‐Wide FINGERS study participants whose contributions make its research possible.
Röhr S, Arai H, Mangialasche F, et al. Impact of the COVID‐19 pandemic on statistical design and analysis plans for multidomain intervention clinical trials: Experience from World‐Wide FINGERS. Alzheimer's Dement. 2021;7:e12143. 10.1002/trc2.12143
Susanne Röhr, Hidenori Arai, and Francesca Mangialasche contributed equally to this article.
REFERENCES
- 1. Heymann DL, Shindo N. WHO Scientific and Technical Advisory Group for Infectious Hazards, COVID‐19: what is next for public health. Lancet. 2020;395:542‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Dorn A. COVID‐19 and readjusting clinical trials. Lancet. 2020;396:523‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Udeh‐Momoh CT, de Jager‐Loots CA, Price G, Middleton LT. Transition from physical to virtual visit format for a longitudinal brain aging study, in response to the Covid‐19 pandemic. Operationalizing adaptive methods and challenges. Trans Res Clin Intervention. 2020;6:e12055. 10.1002/trc2.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Dijk SDM, Bouman R, Folmer EH, et al. Vi)‐rushed into online group schema therapy for older adults by the COVID‐19 outbreak in the Netherlands. Am J Geriatr Psych. 2020;28:983‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sathian B, Asim M, Bannerjee I, et al. Impact of COVID‐19 on clinical trials and clinical research: a systematic review. Nepal J Epidemiol. 2020;10:878‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDermott MM, Newman AB. Preserving clinical trial integrity during the coronavirus pandemic. JAMA. 2020;323:2135‐2136. [DOI] [PubMed] [Google Scholar]
- 7. DeMets DL, Fleming TR. Achieving effective informed oversight by DMSs in COVID clinical trials. J Clin Epidemiol. 2020;126:167‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robb CE, de Jager CA, Ahmadi‐Abhari S, et al. Early COVID‐19 pandemic: a survey of older adults in London, UK. Frontiers Psychiatr. 2020;11:591120. 10.3389/fpsyt.2020.591120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mattioli AV, Sciomer S, Cocchi C, Maffei S, Gallina S. Quarantine during COVID‐19 outbreak: changes in diet and physical activity increase the risk of cardiovascular disease. Nutrit Metab Cardiovasc Dis. 2020;30:1409‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ammar A, Mueller P, Trabelsi K, et al. Psychological consequences of COVID‐19 home confinement: the ECLB‐COVID19 multicenter study. PLoS One. 2020;15(11):e0240204. 10.1371/journal.pone.0240204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kivipelto M, Mangialasche F, Snyder H, et al. World‐Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alz Dementia. 2020;16:1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kivipelto M, Al Solomon, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement. 2013;9(6):657‐665. [DOI] [PubMed] [Google Scholar]
- 13. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255‐2263. [DOI] [PubMed] [Google Scholar]
- 14. Ngandu T, Lehtisalo J, Levalahti E, et al. Recruitment and baseline characteristics of participants in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER)‐a randomized controlled lifestyle trial. Int J Environ Res Public Health. 2014;11(9):9345‐9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strandberg TE, Levalahti E, Ngandu T, et al. Health‐related quality of life in a multidomain intervention trial to prevent cognitive decline (FINGER). Eur Geriatr Med. 2017;8:164‐167. [Google Scholar]
- 16. Marengoni A, Rizzuto D, Fratiglioni L, et al. The effect of a 2‐year intervention consisting of diet, physical exercise, cognitive training, and monitoring of vascular risk on chronic morbidity‐the FINGER Randomized Controlled Trial. J Am Med Dir Assoc. 2018;19(4):355‐360. [DOI] [PubMed] [Google Scholar]
- 17. Kulmala J, Ngandu T, Havulinna S, et al. The effect of multidomain lifestyle intervention on daily functioning in older people. J Am Geriatr Soc. 2019;67(6):1138‐1144. [DOI] [PubMed] [Google Scholar]
- 18. Kivipelto M, Mangialasche F, Ngandu T. WORLD WIDE FINGERS will advance dementia prevention. Lancet Neurol. 2018;17(1):27. 10.1016/S1474-4422(17)30431-3. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization. Global action plan on the public health response to dementia 2017‐2025. Geneva: World Health Organization; 2017. [Google Scholar]
- 20. World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 21. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zülke A, Luck T, Pabst A, et al. de ‐ study protocol of a pragmatic multi‐center cluster‐randomized controlled prevention trial against cognitive decline in older primary care patients. BMC Geriatr. 2019;19(1):203. 10.1186/s12877-019-1212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robert Koch‐Institute . COVID‐19: Fallzahlen in Deutschland und weltweit. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlen.html, accessed October 19, 2020.
- 24. Röhr S, Reininghaus U, Riedel‐Heller SG. Mental wellbeing in the German old age population largely unaltered during COVID‐19 lockdown: results of a representative survey. BMC Geriatr. 2020;20(1):489. 10.1186/s12877-020-01889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barbera M, Mangialasche F, Jongstrac S, et al. Designing an internet‐based multidomain intervention for the prevention of cardiovascular disease and cognitive impairment in older adults: the HATICE Trial. J Alzheimer's Dis. 2018;62:649‐663. [DOI] [PubMed] [Google Scholar]
- 26. Richard E, van Charante EPM, Hoevenaar‐Blom MP, et al. Healthy ageing through internet counselling in the elderly (HATICE): a multinational, randomised controlled trial. The Lanceet Digit Health. 2019;1:E424‐E434. [DOI] [PubMed] [Google Scholar]
- 27. Gill TM, Beavers DP, Guralnick JM, et al. The effect of intervening hospitalizations on the benefit of structured physical activity in promoting independent mobility among community‐living older persons: secondary analysis of a randomized controlled trial. BMC Medicine. 2017;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wadden TA, Neiberg RH, Wing RR. Four‐year weight losses in the Look AHEAD study: factors associated with long‐term success. Obesity. 2011;19:1987‐1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedman LM, Furberg CD, DeMets DL, Reboussin DM, Granger CB. Fundamentals of Clinical Trials 5th Edition. New York: Spinger International Publishing; 2015. [Google Scholar]
- 30. Evans S. When and how can endpoints be changed after initiation of a randomized clinical trial. PLOS Clin Trial. 2007;2(4):e18. 10.1371/journal.pctr.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Getz KA, Zuckerman R, Cropp AB, Hindle AL, Krauss R, Kaitin KI. Measuring the incidence, causes, and repercussions of protocol amendments. Ther Innov Regul Sci. 2011;45:265‐275. [Google Scholar]
- 32. Gaussoin SA, Espeland MA, Beavers DP, et al. Dementia outcomes after addition of proxy‐based assessments for deceased or proxy‐dependent participants. Int J Geriatr Psychiatr. 2019;34:1403‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


