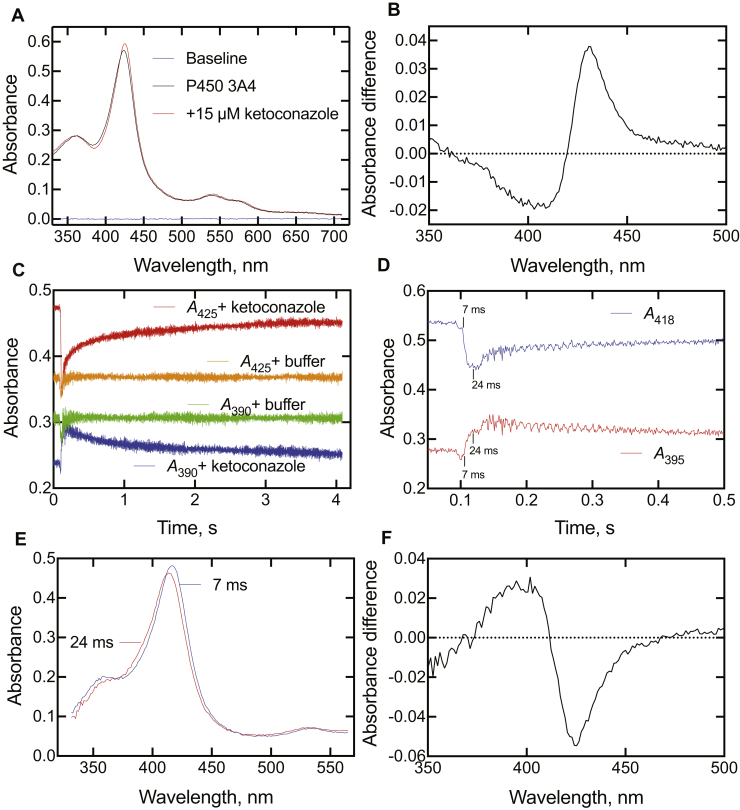
Figure 6.
Absorbance changes at 390 and 425 nm observed upon mixing ketoconazole with cytochome P450s (P450s).A, steady-state spectra of P450 3A4 (5 μM) in the absence and presence of 15 μM ketoconazole (in 100 mM potassium phosphate, pH 7.4). Addition of more ketoconazole did not produce further changes, as would be expected from the submicrometer IC50 (Fig. 2) and Kd values for ketoconazole and P450 3A4. B, difference spectrum from part A, with the P450-only spectrum subtracted from the spectrum obtained in the presence of ketoconazole. C, P450 3A4 (2 μM, final) was mixed with ketoconazole (15 μM). The indicated traces were obtained when the P450s were mixed with only the buffer (100 mM potassium phosphate, pH 7.4). The data were collected in the OLIS Show Pre-trigger Mode, with 0.1 s of data from the previous run (completed reaction) shown prior to actual mixing. D, expansion of data from experiment in part C, with the reaction being observed after 0.1 s (100 ms). The time points (7 and 24 ms) are calibrated for time after the initiation of the reaction (100 ms). E, spectra obtained upon P450 3A4 and ketoconazole 7 and 24 ms after reaction (from parts C and D), with the 7 ms spectrum reflecting mostly unbound P450 and the 24 ms spectrum reflecting the first observed P450·ketoconazole complex. F, difference spectrum generated from part E by subtracting the 7 ms spectrum from the 24 ms spectrum.