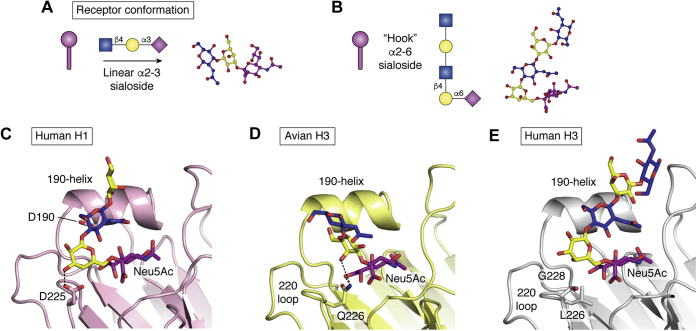
Figure 6.
Receptor-binding adaptations are dictated by the conformation of receptor itself.A, cartoon and isolated receptor structures showing respective low-energy linear and “hook”-shaped conformations of α2-3 and α2-6 sialosides, respectively. C–D, human-adaptive variants favor accommodation of the α2-6-linked receptor conformation and lead to hindrance of linear α2-3 receptors. C, the smaller D190 side chain increases stability and reduces clashing with human-type receptors, while D225 adds a new H-bonding interaction. D, Q226 favors binding of galactose in the linear avian-type conformation through H-bonding interactions. E, mutation of Q226 to leucine (L) removes favorable α2-3-binding H-bonds while the new hydrophobic side chain hinders sugars lying directly over this position. Panel (A) assembled using CCP4Mg (148), panels (C–D) assembled in Pymol (Schrodinger LLC) using PDB IDs: 1RVZ, 1MQM, & 6TZB.