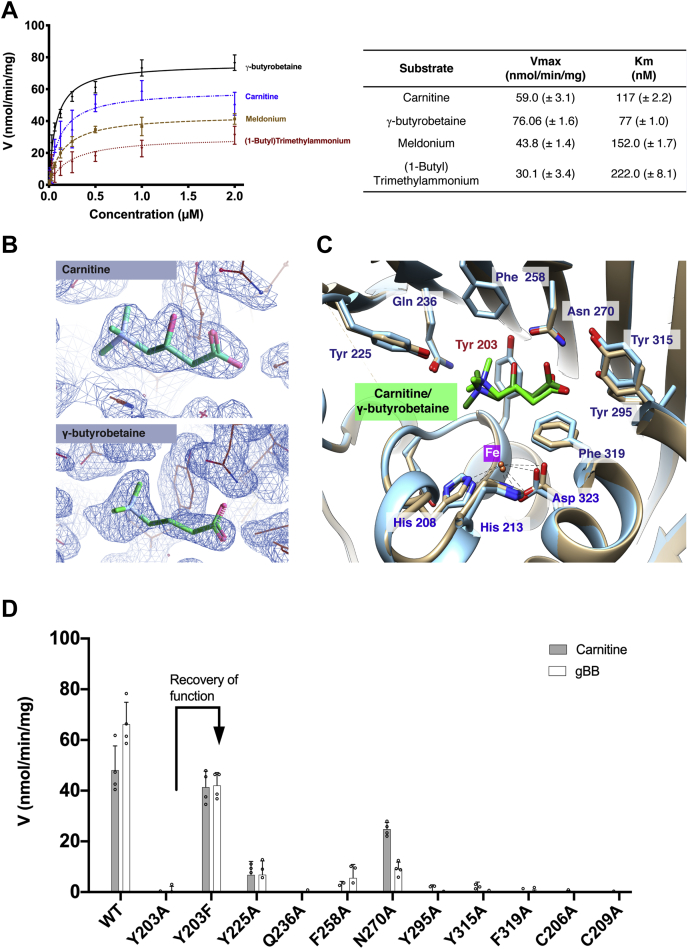
Figure 3.
CntA substrate binding pocket.A, Michaelis–Menten kinetics of CntA (n = 4). B, 2mFo−DFc map at 1.5σ maps (blue) of carnitine displayed at 3.0σ and γ-butyrobetaine (gBB) displayed at 2.5σ. C, carnitine and γ-butyrobetaine interacting residues in the active site among which Y203 interacts with the substrate of CntA through the π–cation interaction. D, enzyme activity of site-directed mutants of key residues involved in substrate coordination in CntA from N = 3 independent replicates. The Y203F mutant in CntA regains activity alluding to the crucial role of the aromatic system for a π–cation interaction.