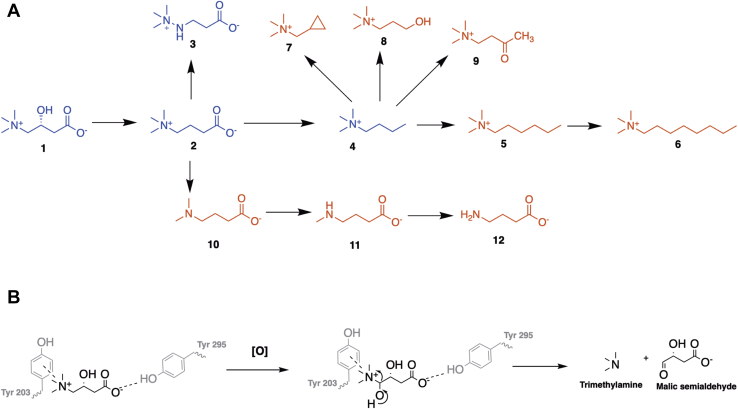
Figure 5.
Structure–activity relationship of CntA substrate profiling and coordination during catalysis.A, a structure–activity relationship scaffold hopping map of substrate analogs tested with a single change between structures and the progression shown with arrows. Structures in blue represent active substrates, whereas those in red are inactive. (1) Carnitine, (2) g-BB, (3) Meldonium, (4) (1-Butyl)Trimethylammonium, (5) (1-Hexyl)Trimethylammonium, (6) (1-Octyl)Trimethylammonium, (7) (Cyclopropylmethyl)-Trimethylammonium, (8) (3-Hydroxypropyl)Trimethylammonium, (9) (3-Oxobutyl)Trimethylammonium, (10) 4-(Dimethylamino)butyric acid, (11) 4-(Methylamino)butyric acid and (12) gamma-Aminobutyric acid. B, the coordination of quaternary amine substrates in the CntA-binding site during catalysis showing the role played by Tyr203 and Tyr295 in coordinating the substrates.