Figure 1.
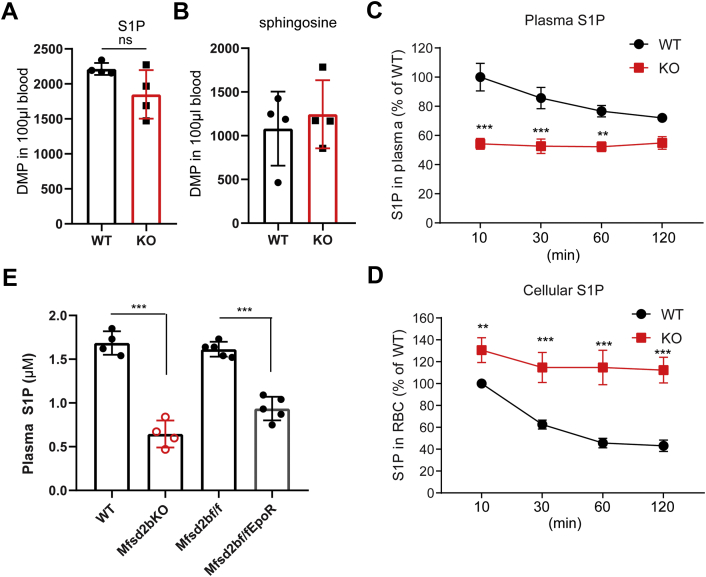
Erythrocytes efficiently import sphingosines for S1P synthesis and release. A, B, radioactive signals of S1P and sphingosine isolated from whole blood (blood cells and plasma) at 1 min after i.v. injection with radioactive sphingosine. There is no significant difference in the amount of S1P produced and sphingosine leftover in whole blood samples between WT and KO at 1 min. Experiments were performed twice with n = 4 to 6 mice for each genotype. C, D, time course of S1P production in plasma and blood cells after IV injection with sphingosine. We noted that exogenous sphingosine was quickly taken up for S1P synthesis and released by red blood cells. The highest level of S1P was detected between 1 and 10 min after sphingosine injection and reduced over the indicated times in WT mice. Intracellular S1P levels from blood cells were also reduced over time, indicative of active export of S1P. Owing to technical limitations, we only collected samples from 10 min onward. Experiments were performed twice with n = 7 to 8 mice per genotype. ∗∗p < 0.01, ∗∗∗p < 0.001; p values were calculated using two-way ANOVA. E, lipidomics analysis of plasma S1P from WT, global KO, Mfsd2bf/f, and Mfsd2bf/fEpoR-Cre mice. Data are mean and SD, n = 4 to 5 per genotypes, ∗∗∗p < 0.001. p Values were calculated using one-way ANOVA. ns, not significant.