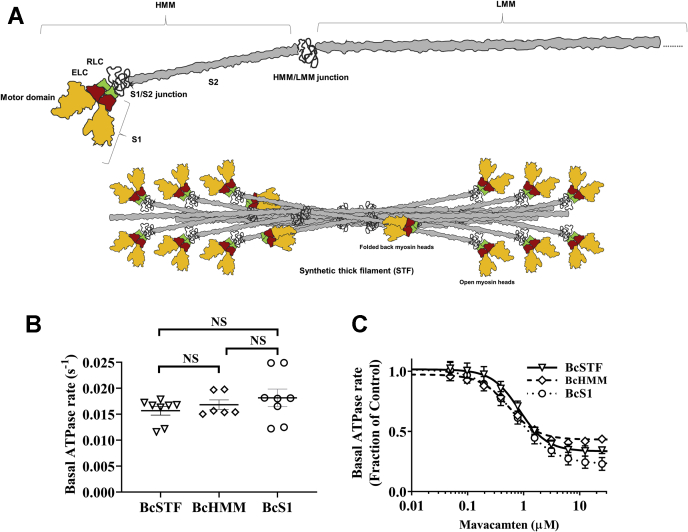
Figure 1.
Basal myosin ATPase activity, as well as potency of mavacamten inhibition in the basal myosin ATPase activity, is similar between BcSTFs, BcHMM, and BcS1.A, the schematic structure of the cardiac myosin with the different subdomains marked (top). The HMM domain of the myosin used in this study consists of the two S1 domains and the S2 domain. The S2 domain of an HMM molecule prepared by chymotryptic cleavage of myosin contains ∼46 heptad repeats. A previous study by Anderson et al. (13) used 2 and 25 heptad versions of the HMM. The unstructured S1–S2 junction separates the S1 and the S2 subfragments of the myosin molecule. Each myosin S1 head consists of a motor domain, ELC, and RLC subunits. LMM is the C-terminal portion of the coiled-coil tail to the right of the HMM–LMM junction. A truncated version of the LMM is shown for illustrative purposes. Full-length myosin self-assembles into bipolar synthetic thick filaments (STFs) in low-ionic-strength buffer (<150 mM), with some myosins in the open-head configuration and others in the folded-back IHM-like state, with the S1 heads folded back onto the S2 tail (bottom). B, absolute basal ATPase rate (in s−1) of BcSTFs, BcHMM, and BcS1 before treatment with mavacamten. C, normalized basal ATPase profiles of BcSTFs, BcHMM, and BcS1 after treatment with increasing concentrations of mavacamten. BcS1 refers to bovine cardiac myosin subfragment-1, BcHMM refers to bovine cardiac heavy meromyosin, and BcSTFs refers to bovine cardiac synthetic thick filaments. The DMSO values were used to normalize the data in the respective untreated systems. The concentrations of mavacamten required for half-maximal change (IC50) estimated from these ATPase curves were 0.76 ± 0.06, 0.57 ± 0.06, and 0.82 ± 0.06 μM for BcS1, BcHMM, and BcSTFs, respectively. Data are expressed as the mean±SEM (n ≥ 6 from at least two experiments). ELC, essential light chain; HMM, heavy meromyosin; IHM, interacting-heads motif; LMM, light meromyosin; NS, not significant; RLC, regulatory light chain; S1, subfragment 1; S2, subfragment 2.