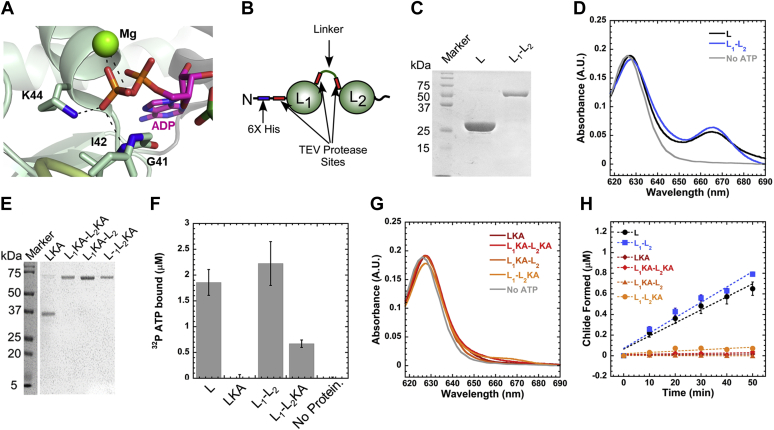
Figure 6.
Both ATP binding sites are required for DPOR function.A, Lys-44 stabilizes nucleotide binding through interactions with the phosphate group of ADP (PDB ID: 3FWY). B, the schematic of the linked-L-protein design and the positions of the TEV protease cleavage sites. C, SDS-PAGE analysis of the purified BchL and linked-BchL-proteins. D, spectroscopic analysis of Pchlide reduction activity of BchL (L, black trace), linked-BchL-protein (L1-L2, blue trace), and no-ATP negative control (no ATP, gray trace). Pchlide absorbance is observed at 625 nm, and Chlide formation is monitored at 665 nm 60 min after incubation with ATP. E, SDS-PAGE analysis of the purified BchLK44A (LKA) and versions of the single and double Lys-44 to Ala–substituted linked-BchL-proteins. F, nitrocellulose filter binding analysis of 32P-ATP binding by WT and Lys-44 to Ala–substituted BchL proteins. L and L1-L2 are capable of binding ATP, whereas the singly substituted L1KA-L2 is partially able to bind ATP. When both subunits are substituted with Lys44 to Ala, no ATP binding is observed. G, linked and unlinked BchL proteins carrying the K44A substitution are incapable of reducing Pchlide. Data shown were collected 60 min after incubation with ATP. H, kinetics of Pchlide reduction measured as a function of Chlide formation is shown. L and L1-L2 reduce Pchlide to Chlide (kobs = 0.01266 ± 0.007 μM·min-1 and 0.0148 ± 0.0022 μM·min−1, respectively). DPOR, dark-operative protochlorophyllide oxidoreductase; Chlide, chlorophyllide; Pchlide, protochlorophyllide; TEV, tobacco etch virus.