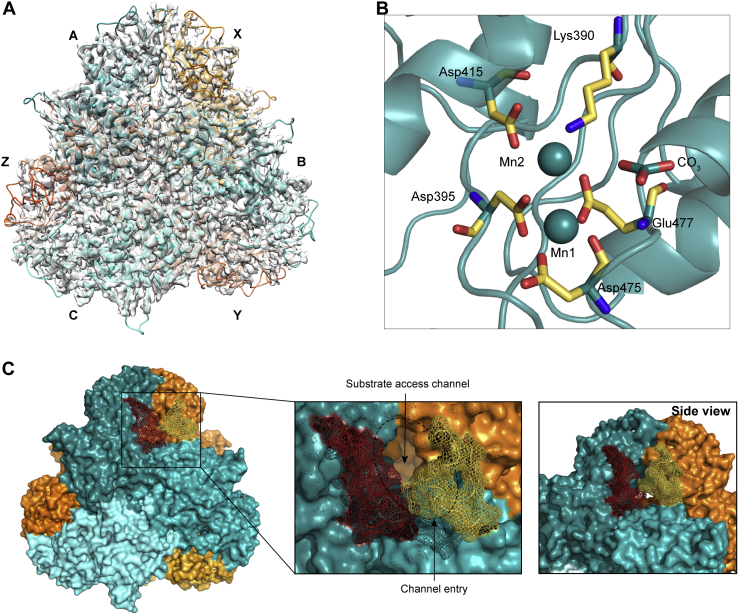
Figure 6.
The full length Pv-M17 cryo-EM structure also adopts the conserved M17 hexameric conformation and has a conserved active site conformation.A, 2.6 Å cryo-EM structure of full length Pv-M17. Cryo-EM structure also adopts the hexameric conformation, consisting of a front trimer depicted in shades of teal and labeled A, B, and C, and a back trimer depicted in shades of orange and labeled X, Y, and Z. B, Pv-M17 full length cryo-EM structure active site. Metal-coordinating residues are shown as yellow sticks, colored by atom. Mn1 and Mn2 are depicted as spheres. Mn1 is coordinated by the carboxyl group of Asp395, Asp475, and Glu477 and the amine group of Asp475. Mn2 is coordinated by the carboxyl group of Asp415, Asp395, Lys390, and Glu477. The active site additionally binds a carbonate ion that is beyond binding proximity to the two zinc ions. C, close up of N-terminal region with Pv loop of chain X (yellow) interacting with the substrate regulation loop of chain A (red) and occluding the active site substrate access channel.PDB ID: 7K5K.