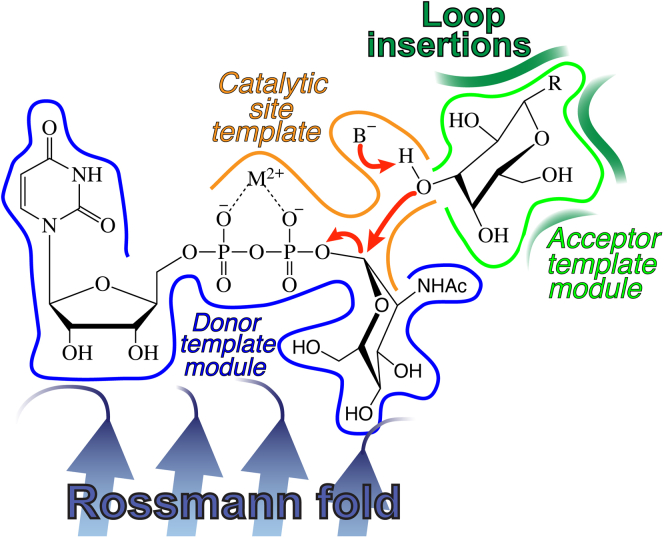
Figure 7.
The modular assembly of the active site of metal-dependent, inverting GT-A fold glycosyltransferases. A generalized model for the active site of a GT-A fold metal-dependent, inverting glycosyltransferase is depicted based on the structure of the proposed B3GNT2 catalytic mechanism (red arrows). Residues in the “Donor template module” (blue outline) facilitate sugar–nucleotide interactions and define the specificity for nucleotide and donor sugar. The “Acceptor template module” (green outline) recruits the extended glycan acceptor and appropriately positions the hydroxyl nucleophile using loop insertions into the core GT-A fold. The “Catalytic site template” (orange outline) positions the catalytic base relative to the C1 of the donor sugar and hydroxyl nucleophile to specify the inverting or retaining mechanism for group transfer.