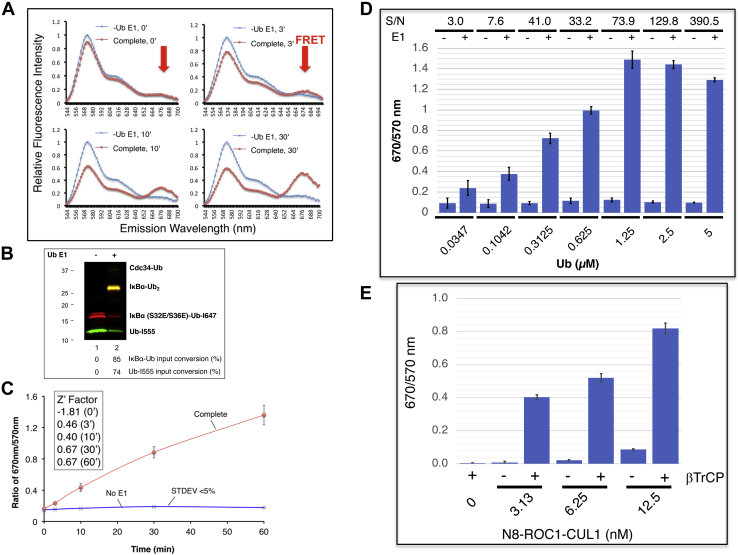
Figure 3.
Tracking FRET IκBα diubiquitination in real time.A, real-time kinetics. The real-time kinetic experiments were carried out as described in Experimental procedures section. The emission spectrum of 544 to 700 nm for the complete reaction and no E1 control are shown. B, gel analysis. The final reaction mixture was subject to SDS-PAGE and Typhoon FLA9500 imaging analysis. C, assessing reliability. Three independent real-time kinetics experiments were carried out, and the results are plotted to reveal standard deviation and calculate Z' factor. The ratio of 670/570 nm represents the FRET efficiency. Note that iFluo 647 (receptor fluorophore) emits at 670 nm, whereas iFluo 555 (donor fluorophore) emits at 570 nm. D, analyte titration. The analyte concentrations are as indicated. The concentrations of enzymes are as follows: E1 (50 nM), E2 Cdc34b (3 μM), and E3 Nedd8–SCFβTrCP (100 nM). S/N = (mean of signal – mean of background)/standard deviation of background. The reaction at 30 °C was monitored on the fluorescence plate reader for 30 min. The 15 min time point (in linear range of the reaction) is used for graph. E, dependency on Skp1–βTrCP and Nedd8–ROC1–CUL1. Skp1–βTrCP (100 nM), donor/receptor Ub (2.5 μM), E2 (50 nM), Cdc34b (1 μM), IκBα (EE)–Ub (E64C)–I647 (2.5 μM), and Ub (K48R/Q31C)–I555 (2.5 μM) were used. The concentrations of Nedd8–ROC1–UL1 are as indicated. The 30 min time point (in linear range of the reaction) is used for graph. Ub, ubiquitin.