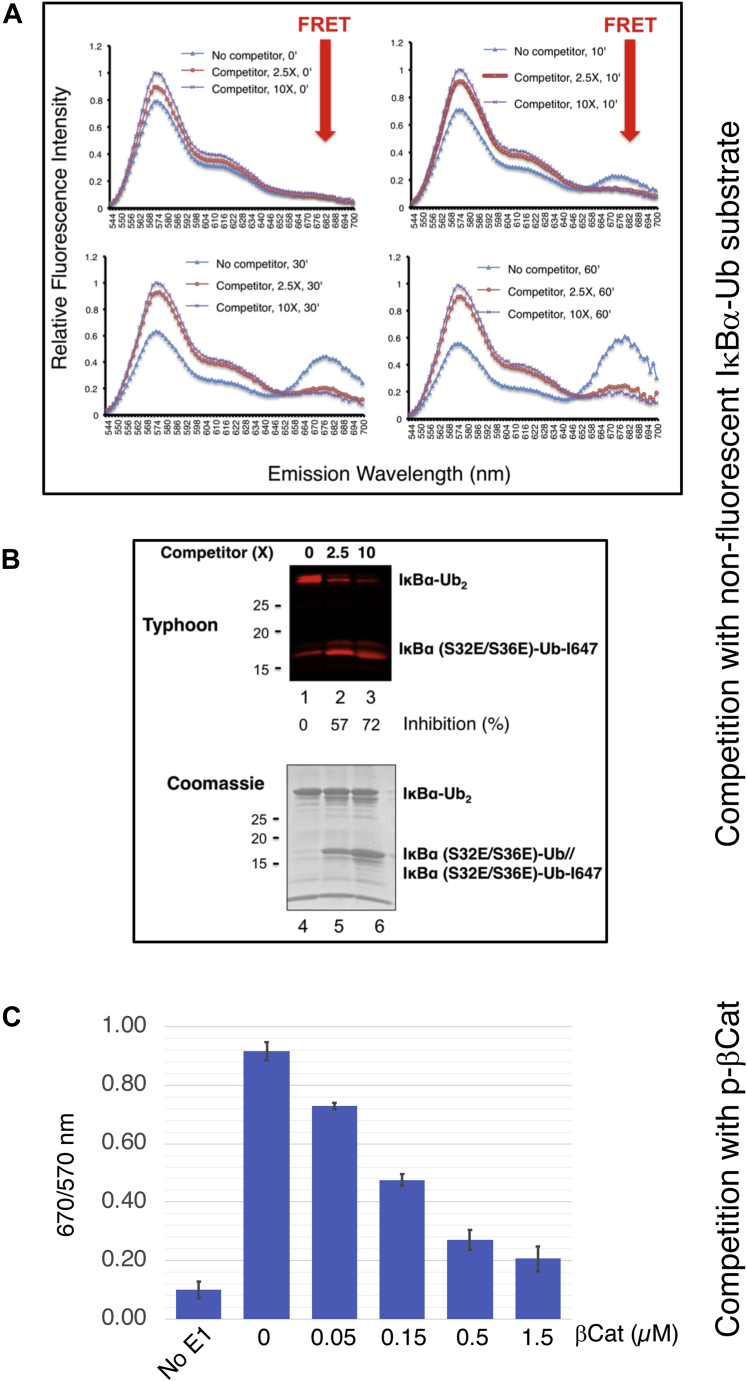
Figure 5.
Real-time kinetics of FRET IκBα diubiquitination with competitor.A–B, the reaction was carried as in Figure 3, A–C, except that nonfluorescent IκBα (EE)–Ub (E64C) was added 2.5× or 10× more than fluorescent IκBα (EE)–Ub (E64C)–I647. The reaction was analyzed by spectroscopic analysis (panel A), and gel electrophoresis followed by imaging (top) or Coomassie stain (bottom). The results showed that fluorescent IκBα–Ub2 product was decreased in the presence of the competitor (top, lanes 1–3). Coomassie staining revealed that nonfluorescent IκBα (EE)–Ub (E64C) was able to support ubiquitination, forming nonfluorescent IκBα–Ub2 (bottom, lanes 4–6). C, competition with β-catenin. The concentrations of enzymes and proteins are as follows: donor/receptor Ub (0.5 μM), E1 (50 nM), E2 Cdc34b (1 μM), Skp1–βTrCP (100 nM), Nedd8–ROC1–CUL1 (12 nM), IκBα (EE)–Ub (E64C)–I647 (0.5 μM), and Ub (K48R/Q31C)–I555 (0.5 μM). The reaction at 30 °C was monitored on the fluorescence plate reader for 30 min. The 18 min time point (in linear range of the reaction) is used for graph. Ub, ubiquitin.