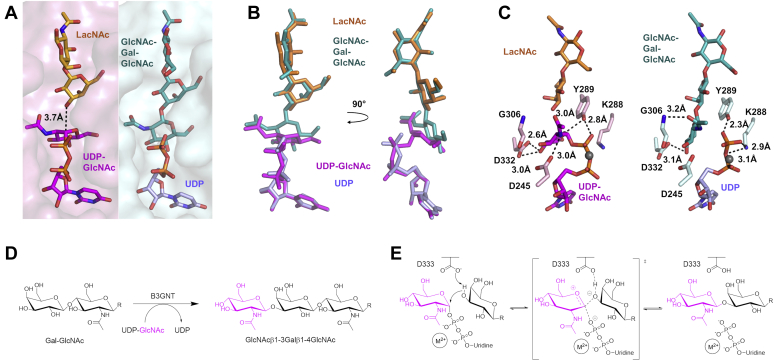
Figure 5.
Mechanism of B3GNT2 glycosyltransferase reaction. (A) Left panel, superposition of B3GNT2_UDPGlcNAc and B3GNT2_LacNAc at the substrate-binding cleft. The surface representation of substrate-binding cleft of B3GNT2_UDPGlcNAc is shown in pink. The distance between the C1 of the GlcNAc in UDP-GlcNAc (magenta) and the hydroxyl group on C3 of the Gal in LacNAc (orange) is marked by dashed line. Right panel, the substrate-binding cleft (cyan surface) in B3GNT2_tri_UDP is shown in the same orientation as the left panel and the two products (GlcNAcβ1-3Galβ1-4GlcNAc in teal and UDP in purple) are shown as sticks. (B) Superposition of the substrates and products in the substrate-binding cleft. UDP-GlcNAc, magenta; LacNAc, orange; GlcNAcβ1-3Galβ1-4GlcNAc, teal; UDP, purple. (C) Change of the hydrogen-bonding interactions in the substrate-binding cleft before (left panel) and after (right panel) the reaction takes place. UDP-GlcNAc, magenta; LacNAc, orange; GlcNAcβ1-3Galβ1-4GlcNAc, teal; UDP, purple; magnesium ion, gray sphere. (D) Glycosyltransferase reaction catalyzed by B3GNT2. (E) Mechanism of a divalent-metal-dependent inverting glycosyltransferase reaction. Asp333 is proposed to be the active site base. The GlcNAc to be transferred is proposed to undergo an oxocarbenium ion-like transition state.