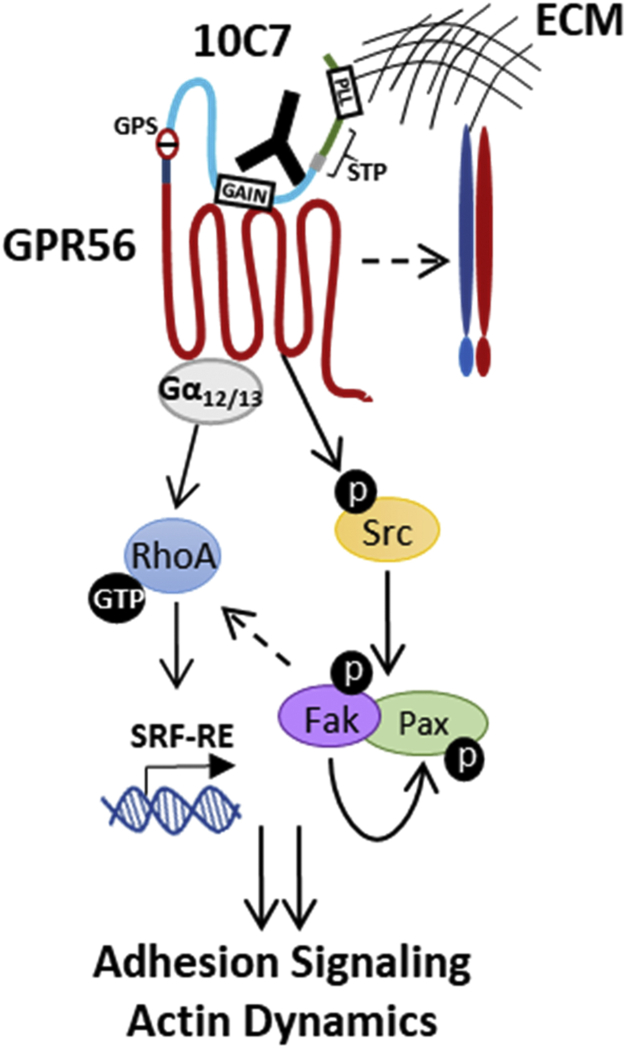
Figure 7.
Schematic of 10C7-induced GPR56 adhesion signaling model. GPR56 activates G⍺12/13-RhoA–SRF signaling and promotes Src phosphorylation independent of RhoA. Src activation leads to phosphorylation of Fak and paxilllin. Binding of 10C7 to the GAIN domain of the ECD induces a conformational change of the ECD and/or promotes the potential interaction with other surface proteins (e.g., integrins) to potentiate Src–Fak signaling and adhesion to the extracellular matrix (ECM). 10C7 potentiation of Src–Fak signaling enhances RhoA–SRF signaling downstream of G12/13 through an unknown mechanism. Deletion of the STP domain suppressed activation of Src–Fak signaling and inhibits 10C7 activity. Truncation of the PLL domain or inhibition of receptor autoproteolysis via mutation of the GPS site decreases constitutive RhoA–SRF signaling, yet is dispensable for 10C7-induced activation of RhoA–SRF or Src–Fak signaling. GPR56 likely coordinates activation of RhoA–SRF and Src–Fak signaling pathways to regulate adhesion and other cellular processes.