ABSTRACT
Background
Reward sensitivity has been proposed as a potential mediator of outcomes for bariatric surgery.
Objectives
We aimed to determine whether gustatory and psychometric measures of reward-related feeding are predictors of bariatric-induced weight loss.
Methods
A multicenter longitudinal cohort study was conducted in patients scheduled for bariatric surgery (surgical group), assessed at baseline and 2 follow-up assessments. Predictions of % weight loss from baseline (%WL) according to baseline gustatory measures, including intensity and pleasantness ratings of sweet and other tastants, and psychometric measures of reward-related feeding behavior, including hedonic hunger scores, were assessed with multivariable linear regression. Exploratory analyses were conducted to test for associations between %WL and changes in gustatory and psychophysical measures, as well as for comparisons with data from patients on the surgery waiting list (control group).
Results
We included 212 patients, of whom 96 in the surgical group and 50 in the control group were prospectively assessed. The groups were similar at baseline and, as expected, bariatric surgery resulted in higher %WL (BTreatment-Time = 2.4; 95% CI: 2.1–2.8; P < 0.0001). While variation in gustatory measures did not differ between groups, in the surgery group baseline sweet intensity predicted %WL at the primary endpoint (11 to 18 months postoperatively; β = 0.2; B = 0.2, 95% CI: 0.02 to 0.3; P = 0.02), as did hedonic hunger scores (β = −0.2; B = −2.0, 95% CI: −3.8 to −0.3; P = 0.02). Furthermore, at this endpoint, postsurgical reduction of sweet taste intensity and acceptance of sweet foods were associated with %WL (β = −0.3; B = −3.5, 95% CI: −5.8 to −1.3; P = 0.003, and β = −0.2; B = −4.7, 95% CI: −8.5 to −0.8; P = 0.02, respectively). The use of sweet intensity as a predictor of weight change was confirmed in another bariatric cohort.
Conclusions
Sweet intensity ratings and hedonic hunger scores predict %WL after surgery. The variability of sweet intensity ratings is also associated with %WL, further suggesting they may reflect physiological processes that are variably modulated by bariatric surgery, influencing clinical outcomes.
Keywords: food reward, feeding behavior, sweet taste, gustation, psychometry, weight loss, bariatric surger
Introduction
Bariatric surgery is the most effective treatment for severe obesity, resulting in weight loss (1, 2), improvement of comorbidities (1, 3), and decreased mortality (1). Gastric bypass (GB) and sleeve gastrectomy (SG) are the most frequently performed bariatric procedures and have similar weight loss outcomes after 5 years (2). However, weight loss is variable (4), with better outcomes in patients adopting healthier behaviors (5) and in those with greater improvement of maladaptive feeding behaviors (6) after surgery. Understanding the mechanisms of such behavioral changes is an opportunity to develop weight loss predictors (7).
The exact nature of the behavioral impact of bariatric surgery on feeding behavior remains elusive (8). Hypotheses span from development of conditioned avoidance to changes in reward-related feeding behavior (8, 9). Indeed, there is evidence of a postoperative decrease in the preference for foods with high fat and sugar (9) and of postoperative changes in measures of implicit (10) and self-reported food reward (11–14), including liking ratings for sucrose-sweetened milk beverages (15). However, it is unclear whether these effects are taste or nutrient dependent or, instead, reflect reduced food reward. In fact, although studies using direct measures of taste function are scarce (16–22), changes in taste perception after surgery have been reported (9). Regarding acuity in taste quality identification or detection/recognition thresholds for sweet tastants, sensitivity was reported to be increased (17–19, 21) or unaltered (16, 20, 22), while responses to suprathreshold intensities revealed unaltered intensity perceptions of sucrose or glucose (16, 20). Such differences in findings regarding the impact of bariatric surgery on taste are likely to be influenced by methodological heterogeneity, with most studies limited by small sample sizes, a lack of nonsurgical control groups, and/or short follow-ups (9). It thus remains unclear whether bariatric surgery induces a fundamental shift in palatability of high-fat and sugary foods or simply decreases appetitive drive for their consumption (9).
It is also unknown whether presurgical ratings of palatable food can be used to predict weight loss after surgery. Wanting, but not liking, for sucrose solutions discriminated weight loss profiles differentially after GB and SG in one study (23). In another study, a GB-specific effect for liking, rather than wanting, scores of sucrose-sweetened milk, as well as reduced responses to sucrose-sweetened milk beverages in the ventral tegmental area, were predictive of increased weight loss (15). However, weight loss predictions according to presurgical self-reported food reward levels are inconsistent (14, 24, 25) and have not been studied for pure measures of sweet taste function. Here, we hypothesized that measures of reward sensitivity—namely, sweet taste perception and self-assessed reward-related feeding behaviors—predict weight loss after bariatric surgery. To address limitations in previous studies, data were collected in a large, multicenter cohort of patients using a pure sweet tastant that was not ingested. Secondary aims included testing associations of weight loss with postoperative changes in these variables and exploring postoperative changes in these variables when compared with a control group, including examining differential effects between the 2 surgery types.
Methods
The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 1983, and was approved by local ethics committees at each of the participating institutions. Written consent was required from all participants.
Study design and subjects
This prospective cohort study included consecutive patients with obesity at 3 Portuguese tertiary care outpatient centers that specialize in surgical treatment of obesity: namely, Hospital do Espírito Santo de Évora, Hospital de São Bernardo de Setúbal, and Centro Hospitalar Universitário de São João. Patients were selected according to approval for bariatric surgery, following the criteria defined in the Portuguese National Health Service (please see Supplementary Material for details), and were recruited over a 4-year period, with data collected from November 2012 to June 2017. The cohort included a main group of patients on the waiting list for bariatric surgery (surgical group), assessed at baseline when surgery was scheduled and twice after surgery in early (3 to 8 months postsurgery) and late (11 to 18 months postsurgery) follow-ups. Since weight loss and feeding behavior are not likely to be stable shortly after surgery, the late follow-up was the main study endpoint. For exploratory comparisons with the surgical group, a control group of patients was recruited at admission to the bariatric surgery waiting list for a baseline assessment and was reassessed at a single follow-up occurring within the periods defined for the surgical group and, necessarily, prior to surgery; that is, patients in the control group did not transition to the surgical group within the study. The 2 groups had similar exclusion criteria, specified in Supplementary Material.
For confirmation of the main findings obtained in our multicentric cohort, data from a distinct bariatric cohort were explored. The latter included post–bariatric surgery patients and contemporaneously recruited healthy volunteers. This cohort was originally recruited for a parallel study that comprised all the psychometric and gustatory measures, collected with the same methodology used in the current study. The exclusion criteria were also the same as applied to the multicentric cohort, with the addition of diabetes mellitus (type 1 or 2). Healthy volunteers were recruited from the community, while post–bariatric surgery patients were recruited from a fourth center specializing in surgical treatment of obesity (Centro Hospitalar de Lisboa Ocidental). Weight measurement was performed at baseline, with psychometric and gustatory measurements, and then reassessed at follow-up in the same conditions (after overnight fasting, using a digital scale). For the minority of participants that were not available for in-person follow-up, weight was self-reported (healthy volunteers) or obtained from clinical records (please see Supplementary Material for details). Patients had their baseline assessment approximately 2.5 years after bariatric surgery (either GB or SG) and weight was reassessed 4 years after. Healthy volunteers were reassessed for weight 5.6 years after baseline.
Primary outcome
Height and weight were measured with a stadiometer and a digital scale, respectively, at baseline and follow-up by a research technician. BMI was calculated as weight in kilograms divided by height in meters squared. The primary outcome (dependent variable) was weight loss, expressed as the percentage of weight change from baseline [%WL = (weight lost/baseline weight) × 100] (26). The late follow-up (11 to 18 months) was the main study endpoint.
Gustatory and psychometric variables
Multiple gustatory and psychometric measures (independent variables) were assessed to test associations with the primary outcome. A psychometric assessment of reward-related feeding behavior was performed with the Power of Food Scale (PFS) for hedonic hunger (27–29) and the Yale Food Addiction Scale (YFAS) for addiction-like feeding behavior (30, 31). The Dutch Eating Behavior Questionnaire (DEBQ) assessed feeding behavior traits (32, 33), while acceptance for food (fruits, vegetables, dairy, meat, fried, sauces, carbs, sweets) and alcohol was assessed using the Food Action Rating Scale (FARS) (34). Depressive symptom severity was assessed with the Beck Depression Inventory II (35). The taste test consisted of filter paper strips impregnated with deionized water or a solution of 1 of 4 basic tastants—namely, citric acid (sour), sodium chloride (salty), sucrose (sweet), or quinine hydrochloride (bitter)—each of which presented in 4 increasing concentrations (36). For each strip, general labeled magnitude and hedonic scales were used for ratings of intensity (0 to 100) and pleasantness (−100 to 100), respectively (37, 38). Mean intensity and pleasantness ratings for each basic taste quality in each individual were determined across the 4 concentrations of each tastant. To determine taste acuity, taste quality was identified in a 5-item forced-choice test (sour, salt, sweet, bitter, or none of the above). Each correct answer equaled to 1 point, corresponding to a maximum of 4 points for each taste quality score and 16 points for the whole acuity score, as previously performed in prospective bariatric studies (17, 18).
Individual taste thresholds were assessed with electrogustometry (39) using a commercially available electrogustometer. A detailed description of the gustatory protocol is provided in Supplementary Material. According to our hypothesis, the primary independent variables were the mean intensity and pleasantness ratings across 4 concentrations of sucrose (sweet), as well as scores for hedonic hunger and addiction-like feeding behavior. The remaining gustatory and psychometric variables were included as secondary independent variables.
Covariates assessment
For both surgical and control groups, information on age, gender, personal history of type 2 diabetes mellitus (T2DM), and surgical center was obtained at baseline.
Data analysis
Categorical variables are represented as percentages. Continuous variables are represented as means with SDs or 95% CIs. For scales where this is considered admissible (PFS, DEBQ, and FARS), simple imputation with the mean score of the respective subscale was used to address missing data when missing values represented 10% or less of the total items in that subscale. For unadjusted comparisons between groups, an independent-samples t-test, an ANOVA with Bonferroni post hoc testing, or a chi-squared test (χ2) was used, as appropriate.
In preliminary exploratory analyses, to assess the effects of bariatric surgery when compared to medical treatment for obesity, we performed linear mixed-effects longitudinal models (LM-ELM) with each gustatory or feeding behavior variable or with BMI as dependent variables and with treatment (surgery compared with medical) and time in months as the independent variables of interest, while controlling for age, gender, baseline BMI, personal history of T2DM, and surgical center. In each model, an interaction term between treatment and follow-up time in months (BTreatment-Time) was added to assess the difference between the 2 treatment groups in the change of each dependent variable across follow-up time. To allow for more flexible model fitting of LM-ELM, we used randomly varying intercepts and slopes and did not consider any specific structure for the residuals. Multiple linear regression (MLR) models, adjusted for previously defined predictors of bariatric weight loss (age, gender, baseline BMI, and T2DM) (7), as well as surgical center and surgery type, were used to test our main hypothesis regarding predictions of %WL according to baseline reward-related gustatory or psychometric measures. Standardized beta (β) and unstandardized beta (B) coefficients with 95% CIs were presented for these MLR models, and the effect size (R2) of the overall model was provided as a measure of effect size. Assumptions for using MLR and LM-ELM models—namely, linearity and homoscedasticity—were assessed by inspection of the standardized residuals versus predicted values plots. Additionally, for MLR models, we assessed the independence of variables (Durbin-Watson statistic) and the absence of influential points (Cook's distance measure), and residuals were checked for normal distribution by inspecting histograms of standardized residuals.
For our main analyses—namely, MLR models of weight loss prediction according to baseline variables—power analyses were performed a posteriori. These analyses were performed according to the effect size of the overall model (R2), the number of covariables, and the sample size, to estimate the power for that regression, considering α = 0.05. Also for the main analyses, correction for multiple comparisons was performed within each group of variables [intensity ratings, pleasantness ratings, other taste assessment variables, hedonic hunger scores, food addiction variables, feeding behavior traits (DEBQ scores), and food acceptance (FARS scores)] according to Benjamini-Hochberg (40), assuming a false discovery rate (FDR) of 0.1. The same approach was used within the prespecified primary independent variables (mean sweet intensity and mean sweet pleasantness ratings; hedonic hunger and addiction-like feeding behavior scores).
In MLR models of weight loss prediction, differential predictions of %WL according to surgery type were further explored by an interaction term between surgery type and each gustatory or feeding behavior predictor, in the respective regression. For significant interactions, the regressions were then repeated separately for SG and GB groups. Further exploratory analyses were conducted to assess %WL predictors in the control group, as well as associations between %WL and postoperative changes in gustatory or feeding behavior measures, separately for the surgical and control groups. For these analyses, longitudinal changes in gustatory or psychometric measures were determined by normalizing (dividing) follow-up by baseline values. To allow for such computations, variables with negative or null (0) values were transformed by adding the amount needed for the minimum value to be 1 (intensity ratings: +1; pleasantness ratings: +101). Weight loss predictions were further explored in cohorts from a separate study, for confirmatory purposes, using Pearson's correlation (r).
Statistical analyses were performed using SPSS version 25 (SPSS Inc.) or Stata Statistical Software, Release 15 (StataCorp LLC). Graphs were produced using Stata Statistical Software, GraphPad Prism version 8.0 (GraphPad Software), and Adobe Illustrator version CC 2019 (Adobe Inc.). A 2-tailed P value of 0.05 was selected as the significance level for all analyses.
Results
Baseline and follow-up assessments
Of the 313 patients assessed for participation, 212 were eligible and consented to participate: 116 in the surgical group and 96 in the control group. The 2 groups did not differ significantly at baseline, with the exception of a small difference for emotional eating (Table 1) that was no longer significant when only patients with complete follow-up were analyzed (Supplementary Tables 1 and 2). In the control group, 50 patients completed a single follow-up visit 2 to 18 months after baseline, while in the surgical group 96 patients were assessed at early (3 to 8 months; n = 91) and/or late (11 to 18 months; n = 86) follow-ups after surgery (Figure 1). The time at the end of follow-up was, on average, 7.3 (4.3) months for the control group and 12 (2.3) months for the surgical group (mean difference, −4.6 months; 95% CI, −5.7 to −3.5; t144 = −8.4; P < 0.0001). When comparing patients in the surgical group treated with SG or GB, neither baseline variables (Supplementary Table 3) nor follow-up time (mean difference, 0.4 months; 95% CI, −0.5 to 1.4; t94 = 0.9; P = 0.4) were significantly different between the subgroups. Missing data for weight were 0% at all time points, while for gustatory and psychometric variables, missing data ranged from 0.9 to 8.6% at baseline and 2.1 to 7.3% at follow-up.
TABLE 1.
Baseline characteristics of patients in control and surgical groups1
| Baseline variable | Control, n = 96 | Surgical, n = 116 | P value2 |
|---|---|---|---|
| Age, years | 43.1 ± 10.1 | 43.3 ± 10.3 | 0.9 |
| Women | 81 (84.4) | 99 (85.3) | 0.8 |
| Education, years | 10.4 ± 4.1 | 10 ± 4.1 | 0.5 |
| T2DM | 22 (22.9) | 20 (17.2) | 0.3 |
| Smokers | 24 (25.0) | 22 (19.0) | 0.3 |
| BMI, kg/m2 | 42.1 ± 5.2 | 43.1 ± 5.4 | 0.2 |
| BDI-II score | 13.5 ± 11.1 | 12.6 ± 10.6 | 0.5 |
| Taste thresholds, dB | 18.2 ± 14.1 | 18.6 ± 14.9 | 0.9 |
| Acuity | 12.2 ± 2.8 | 12.7 ± 2.7 | 0.2 |
| Sour ratings, mm | |||
| Intensity | 54.2 ± 19.6 | 53.8 ± 18.4 | 0.9 |
| Pleasantness | −39.8 ± 31.2 | −38.1 ± 28.4 | 0.7 |
| Salt ratings, mm | |||
| Intensity | 28.8 ± 13.0 | 30.7 ± 13.7 | 0.3 |
| Pleasantness | −11.3 ± 18.0 | −9.4 ± 17.2 | 0.5 |
| Sweet ratings, mm | |||
| Intensity | 20.4 ± 10.6 | 22.7 ± 14.8 | 0.2 |
| Pleasantness | 11.1 ± 11.9 | 13.8 ± 17.1 | 0.2 |
| Bitter ratings, mm | |||
| Intensity | 45.2 ± 20.5 | 43.2 ± 16.7 | 0.4 |
| Pleasantness | −41.4 ± 24.7 | −36.4 ± 20.8 | 0.1 |
| Reward-related feeding behavior | |||
| PFS – Aggregate score | 2.3 ± 0.8 | 2.3 ± 0.9 | 0.8 |
| PFS – Food Available | 2.1 ± 0.9 | 2.1 ± 0.9 | 0.7 |
| PFS – Food Present | 2.7 ± 1.0 | 2.7 ± 1.2 | 0.7 |
| PFS – Food Tasted | 2.3 ± 0.8 | 2.5 ± 0.9 | 0.2 |
| YFAS – Diagnosis | 21 (23.1) | 27 (23.9) | 0.9 |
| YFAS – No. of symptoms | 2.9 ± 1.8 | 2.5 ± 1.7 | 0.1 |
| Feeding behavior traits | |||
| DEBQ – External Eating | 2.5 ± 0.7 | 2.5 ± 0.7 | 0.9 |
| DEBQ – Restrained Eating | 3.0 ± 0.7 | 3.1 ± 0.7 | 0.4 |
| DEBQ – Emotional Eating | 2.4 ± 0.9 | 2.1 ± 0.8 | 0.04 |
| Food acceptance | |||
| FARS – Aggregate score | 409.4 ± 54.7 | 410.5 ± 54.0 | 0.9 |
| FARS – Fruits | 70.8 ± 10.8 | 71.3 ± 12.0 | 0.8 |
| FARS – Vegetables | 102.0 ± 19.7 | 102.8 ± 18.3 | 0.8 |
| FARS – Dairy | 21.4 ± 4.0 | 21.3 ± 4.5 | 0.9 |
| FARS – Meat | 38.4 ± 6.0 | 38.4 ± 7.3 | 1.0 |
| FARS – Fried | 25.4 ± 8.4 | 25.5 ± 7.4 | 0.9 |
| FARS – Sauces | 20.1 ± 6.5 | 19.9 ± 6.1 | 0.8 |
| FARS – Carbs | 61.9 ± 10.3 | 63.6 ± 10.8 | 0.3 |
| FARS – Sweets | 35.1 ± 9.6 | 34.3 ± 8.8 | 0.6 |
| FARS – Alcohol | 8.3 ± 5.3 | 9.2 ± 5.6 | 0.3 |
Results are presented as mean ± SD or number (%). Control data are from patients on the waiting list for bariatric surgery; surgical data are from patients scheduled for sleeve gastrectomy or gastric bypass.
Independent-sample t tests were performed for continuous variables and χ2 tests were performed for categorical variables for comparisons between control and surgical groups.
BDI-II, Beck Depression Inventory II; DEBQ, Dutch Eating Behavior Questionnaire; FARS, Food Action Rating Scale; PFS, Power of Food Scale; T2DM, type 2 diabetes mellitus; YFAS, Yale Food Addiction Scale.
FIGURE 1.
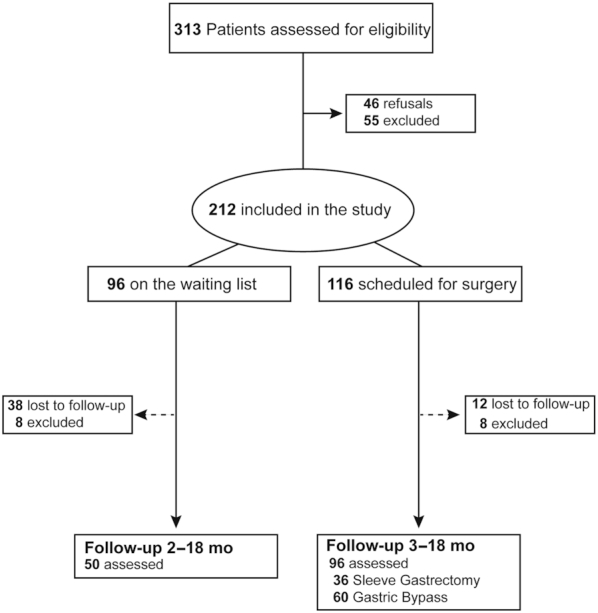
Flow diagram for the study. The follow-up assessments in the surgical group were performed at 2 time points, with 81 patients assessed at both points and the remaining 15 assessed only once after surgery.
Effects of surgery
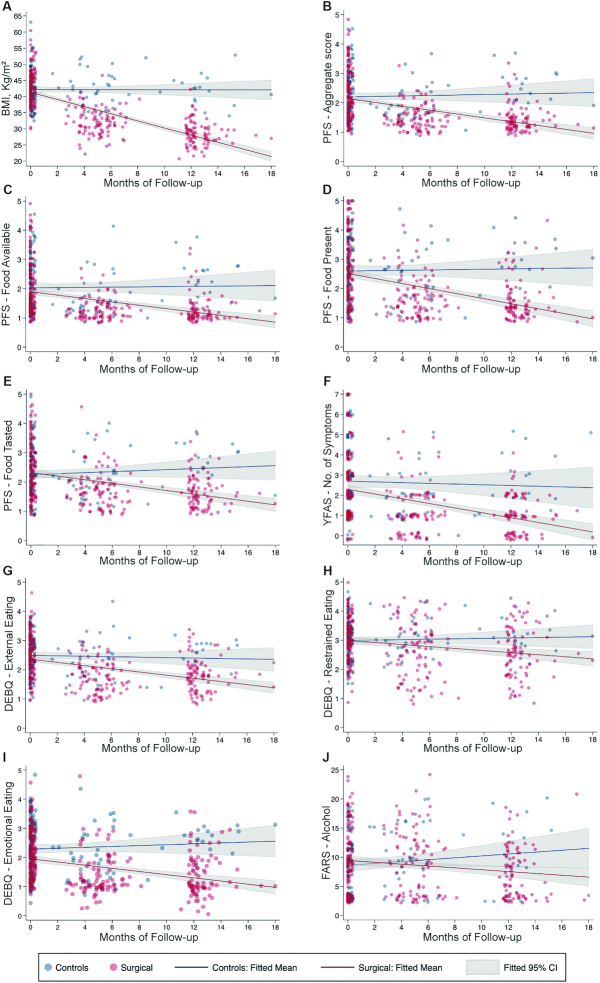
Preliminary exploratory analyses were conducted to compare the surgical and control groups across follow-up. At the end of follow-up, %WL was 31.9% (8.2%) in the surgical and 1% (5.7%) in the control group, with a significant interaction between treatment group and follow-up time in the LM-ELM model for %WL, adjusted for age, gender, baseline BMI, personal history of T2DM, and surgical center (BTreatment-Time = 2.4; 95% CI, 2.1–2.8; P < 0.0001; Supplementary Table 4). This demonstrated induction of weight loss by surgery across time, as expected, which was confirmed in the model for BMI (BTreatment-Time = −1.1; 95% CI: −1.2 to −0.9; P < 0.0001; Figure 2 and Supplementary Table 5). Equivalent evidence for an effect of surgery across time, not observed in the control group, was found in LM-ELM models for hedonic hunger (PFS – Aggregate score: BTreatment-Time = −0.1; 95% CI: −0.01 to −0.04; P < 0.0001; PFS – Food available: BTreatment-Time = −0.1; 95% CI: −0.1 to −0.04; P = 0.01; PFS – Food present: BTreatment-Time = −0.1; 95% CI: −0.1 to −0.04; P < 0.0001; and PFS – Food tasted: BTreatment-Time = −0.1; 95% CI: −0.1 to −0.04; P < 0.0001), food addiction–like symptoms (YFAS – No. of symptoms: BTreatment-Time = −0.1; 95% CI: −0.2 to −0.02; P = 0.01), and feeding behavior traits (DEBQ – Emotional eating: BTreatment-Time = −0.1; 95% CI: −0.1 to −0.03; P < 0.001; DEBQ – Restrained eating: BTreatment-Time = −0.1; 95% CI: −0.1 to −0.01; P = 0.02; DEBQ – External eating: BTreatment-Time = −0.04; 95% CI: −0.1 to −0.01; P = 0.01), as well as acceptance for alcohol (FARS – Alcohol: BTreatment-Time = −0.3; 95% CI: −0.6 to −0.1; P = 0.01; Figure 2 and Supplementary Table 5). Changes in gustatory measures, however, did not differ between the control or surgery groups across follow-ups (Supplementary Table 5). When comparing %WL between surgical interventions at the end of follow-up, SG and GB lost an average of 30.1% (9.7%) and 33.4% (7.6%), respectively, with a significant interaction between surgery type and months of follow-up for %WL (BTreatment-Time = 0.4; 95% CI: 0.01–0.7; P < 0.05; Supplementary Table 4), but not reward-related feeding behavior, feeding behavior traits, food acceptance, or gustatory measures (Supplementary Table 6).
FIGURE 2.
Variables that significantly changed across treatment groups at the end of follow-up. Here, we represent the fitted linear regression lines and respective 95% CIs for linear mixed-effects longitudinal models to assess the effect of bariatric surgery, when compared to medical treatment for obesity, on gustatory and psychometric measures of feeding behavior across follow-up. These models were adjusted for age, gender, personal history of type 2 diabetes mellitus, baseline BMI, and surgical center. For data visualization purposes, in each panel, the representation of individual assessments is jittered around the respective follow-up moment (in months). Only models with significant interactions between time and treatment group are represented. (A) BMI (BTreatment-Time = −1.1; 95% CI: −1.2 to −0.9; P < 0.0001). (B) PFS – Aggregate score (BTreatment-Time = −0.1; 95% CI: −0.01 to −0.04; P < 0.0001). (C) PFS – Food available (BTreatment-Time = −0.1; 95% CI: −0.1 to −0.02; P = 0.01). (D) PFS – Food present (BTreatment-Time = −0.1; 95% CI: −0.1 to −0.04; P < 0.0001). (E) PFS − Food tasted (BTreatment-Time = −0.1; 95% CI: −0.1 to −0.04; P < 0.0001). (F) YFAS – No. of symptoms (BTreatment-Time = −0.1; 95% CI: −0.2 to −0.02; P = 0.01). (G) DEBQ – External eating (BTreatment-Time = −0.04; 95% CI: −0.1 to −0.01; P = 0.01). (H) DEBQ – Restrained eating (B Treatment-Time = −0.1; 95% CI: −0.1 to −0.01; P = 0.02). (I) DEBQ – Emotional eating (BTreatment-Time = −0.1; 95% CI: −0.1 and −0.003; P < 0.001). (J) FARS – Alcohol (BTreatment-Time = −0.3; 95% CI: −0.6 to −0.1; P = 0.01). Control data were from patients on the waiting list for bariatric surgery (n = 50); surgical data were from patients scheduled for bariatric surgery (n = 96). DEBQ, Dutch Eating Behavior Questionnaire; FARS, Food Action Rating Scale; PFS, Power of Food Scale; YFAS, Yale Food Addiction Scale.
Weight loss prediction
The main objective of this study was to identify predictors of weight loss after bariatric surgery. In Table 2, we show analyses testing preoperative gustatory and reward-related feeding behavior variables as predictors of subsequent %WL, 11 to 18 months after surgery, in linear regression models adjusted for baseline BMI, baseline age, gender, personal history of T2DM, surgical center, and surgery type. Since the exact moment of late follow-up (ranging from 11 to 18 months) could be associated with the degree of %WL, in preliminary analyses we tested the association between these variables, which revealed a very small and nonsignificant correlation (r = 0.03; P = 0.8), supporting joint assessment of the data within this range of follow-up times.
TABLE 2.
Weight loss prediction or associations with weight loss at 11 to 18 months, according to gustatory and psychometric measures of reward-related feeding behavior in the surgical group
| Variable: baseline/follow-up change | %WL prediction by baseline variables,1n = 86 | Associations between follow-up change and %WL,2n = 86 | ||
|---|---|---|---|---|
| β (B, 95% CI)3 | P value4 | β (B, 95% CI)3 | P value | |
| Taste thresholds | 0.02 (0.01, −0.1 to 0.1) | 0.8 | −0.1 (−1.3, −3.1 to 0.6) | 0.2 |
| Acuity | −0.1 (−0.4, −1.1 to 0.2) | 0.2 | 0.1 (2.9, −3.8 to 9.7) | 0.4 |
| Sour ratings | ||||
| Intensity | 0.1 (0.03, −0.1 to 0.1) | 0.6 | −0.1 (−3.0, −7.4 to 1.5) | 0.2 |
| Pleasantness | 0.04 (0.01, −0.1 to 0.1) | 0.7 | −0.01 (−0.1, −1.3 to 1.2) | 0.9 |
| Salt ratings | ||||
| Intensity | −0.02 (−0.01, −0.1 to 0.1) | 0.8 | −0.01 (−0.2, −3.3 to 2.9) | 0.9 |
| Pleasantness | 0.1 (0.04, −0.1 to 0.1) | 0.5 | −0.2 (−6.0, −12.9 to 0.9) | 0.1 |
| Sweet ratings | ||||
| Intensity | 0.2 (0.2, 0.02 to 0.3) | 0.02 | −0.3 (−3.5, −5.8 to −1.3) | 0.003 |
| Pleasantness | 0.1 (0.1, −0.1 to 0.2) | 0.4 | −0.1 (−6.8, −16.6 to 2.9) | 0.2 |
| Bitter ratings | ||||
| Intensity | 0.1 (0.1, −0.1 to 0.2) | 0.3 | <0.001 (−0.001, −3.6 to 3.6) | 1.0 |
| Pleasantness | −0.2 (−0.1, −0.2 to 0.01) | 0.1 | 0.1 (0.9, −2.2 to 3.9) | 0.6 |
| Reward-related feeding behavior | ||||
| PFS – Aggregate score | −0.2 (−2.0, −3.8 to −0.3) | 0.02 | −0.003 (−0.1, −7.1 to 6.9) | 1.0 |
| PFS – Food available | −0.2 (−1.9, −3.5 to −0.3) | 0.02 | 0.1 (2.1, −3.8 to 8.0) | 0.5 |
| PFS – Food present | −0.2 (−1.2, −2.7 to 0.2) | 0.1 | −0.04 (−1.0, −5.9 to 3.8) | 0.7 |
| PFS – Food tasted | −0.1 (−0.9, −2.5 to 0.6) | 0.2 | −0.1 (−2.6, −8.0 to 2.9) | 0.4 |
| YFAS – Diagnosis | −0.2 (−3.5, −7.2 to 0.2) | 0.1 | — | — |
| YFAS – No. of symptoms | −0.1 (−0.7, −1.6 to 0.3) | 0.2 | 0.03 (0.5, −3.3 to 4.4) | 0.8 |
| Feeding behavior traits | ||||
| DEBQ – External eating | −0.2 (−2.0, −4.2 to 0.2) | 0.1 | −0.02 (−0.8, −7.4 to 5.9) | 0.8 |
| DEBQ – Restrained eating | −0.1 (−0.6, −2.8 to 1.6) | 0.6 | −0.05 (−1.2, −6.4 to 4.0) | 0.7 |
| DEBQ – Emotional eating | −0.1 (−1.0, −2.8 to 0.8) | 0.3 | −0.2 (−5.0, −10.0 to 0.03) | 0.1 |
| Food acceptance | ||||
| FARS – Aggregate score | −0.1 (−0.02, −0.05 to 0.01) | 0.2 | −0.1 (−6.2, −18.3 to 6.0) | 0.3 |
| FARS – Fruits | 0.1 (0.05, −0.1 to 0.2) | 0.5 | −0.1 (−7.7, −18.5 to 3.2) | 0.2 |
| FARS – Vegetables | −0.1 (−0.04, −0.1 to 0.1) | 0.5 | 0.1 (4.3, −7.9 to 16.4) | 0.5 |
| FARS – Dairy | −0.03 (−0.1, −0.4 to 0.3) | 0.7 | −0.2 (−6.7, −14.7 to 1.2) | 0.1 |
| FARS – Meat | −0.2 (−0.2, −0.4 to 0.04) | 0.1 | −0.1 (−5.8, −14.2 to 2.7) | 0.2 |
| FARS – Fried | −0.2 (−0.2, −0.4 to 0.04) | 0.1 | 0.01 (0.2, −3.7 to 4.1) | 0.9 |
| FARS – Sauces | −0.2 (−0.2, −0.5 to 0.1) | 0.1 | 0.02 (0.6, −4.3 to 5.5) | 0.8 |
| FARS – Carbs | −0.2 (−0.1, −0.3 to 0.02) | 0.1 | −0.1 (−4.2, −11.3 to 2.9) | 0.2 |
| FARS – Sweets | −0.1 (−0.1, −0.3 to 0.1) | 0.4 | −0.2 (−4.7, −8.5 to −0.8) | 0.02 |
| FARS – Alcohol | −0.1 (−0.1, −0.4 to 0.2) | 0.6 | −0.01 (−0.1, −2.5 to 2.4) | 1.0 |
General linear models were adjusted for baseline BMI, baseline age, gender, personal history of type 2 diabetes mellitus, surgical center, and surgery type and were performed to explain %WL from surgery at the end of follow-up. DEBQ, Dutch Eating Behavior Questionnaire; FARS, Food Action Rating Scale; FDR, false discovery rate; PFS, Power of Food Scale; YFAS, Yale Food Addiction Scale.
1%WL according to baseline gustatory and feeding behavior variables.
2%WL follow-up changes in baseline gustatory and feeding behavior variables. Follow-up changes were determined by normalizing follow-up by baseline values. End of follow-up was 11 to 18 mo after surgery.
3Values are standardized beta coefficients (β) and unstandardized beta coefficients (B) with 95% CIs. The overall effect size (R2) of models, both according to baseline variables or follow-up change, ranged from 0.35 to 0.43.
4For these primary analyses, statistical significance was defined using a FDR of 0.1, according to Benjamini-Hochberg (40).
Hedonic hunger was a negative predictor of %WL (PFS – Aggregate score: β = −0.2; B = −2.0, 95% CI: −3.8 to −0.3; P = 0.02; and PFS – Food available: β = −0.2; B = −1.9, 95% CI: −3.5 to −0.3; P = 0.02; R2 = 0.4 for both models), while the mean sweet intensity rating was a positive predictor of %WL (β = 0.2; B = 0.2, 95% CI: 0.02–0.3; P = 0.02; R2 = 0.4). These results remained significant after correction for multiple comparisons, as defined in the Methods section. In several of these models, age, male gender, and T2DM were significant negative predictors and preoperative BMI was a positive predictor of %WL. For the MLR models comprising baseline sweet intensity ratings, the PFS – Aggregate score, or the PFS – Food available score as predictors of %WL, a power of 100% was estimated (see Methods for details).
In exploratory analyses, to test whether surgery type modulated these predictive associations, interactions between surgery type and each predictor were tested in the respective regressions (Supplementary Table 7). For variables with significant interactions with surgery type, separate regressions were performed for each surgical group, revealing several predictors of %WL that were significant only for the GB group: namely, hedonic hunger (PFS – Food Tasted: β = −0.3; B = −2.3, 95% CI: −4.0 to −0.6; P = 0.01), addiction-like feeding behavior (YFAS – No. of symptoms: β = −0.4; B = −1.4, 95% CI: −2.4 to −0.5; P = 0.003), external eating (DEBQ – External eating: β = −0.3; B = −3.1, 95% CI: −5.5 to −0.8; P = 0.01), and acceptance for sauces (FARS – Sauces: β = −0.3; B = −0.3, 95% CI: −0.6 to −0.06; P = 0.02) and alcohol (FARS – Alcohol: β = −0.3; B = −0.4, 95% CI: −0.7 to −0.01; P < 0.05; Supplementary Table 8). Regarding associations with follow-up changes, only reductions in hedonic hunger were associated with the amount of weight lost in the SG group (PFS – Food tasted: β = −0.3; B = −10.3, 95% CI: −20.4 to −0.2; P < 0.05). In exploratory analyses performed in the control group, a different set of variables—namely, feeding behavior traits and acceptance of several foods, including sweet foods and carbohydrates—significantly predicted %WL (Supplementary Table 9).
Association between weight loss and postoperative changes in reward-related measures
Additional exploratory analyses were conducted to assess associations between weight loss and changes in gustatory and psychometric variables, in similarly adjusted linear regression models. At 11 to 18 months after surgery, %WL was associated with decreases in both the mean sweet intensity rating (β = −0.3; B = −3.5, 95% CI: −5.8 to −1.3; P = 0.003) and sweet foods acceptance (β = −0.2; B = −4.7, 95% CI: −8.5 to −0.8; P = 0.02; Table 2). No variation in any of the other gustatory and psychometric variables was significantly associated with %WL. Again, age, male gender, T2DM, and preoperative BMI were significantly associated with %WL in several of these models. Although there were significant interactions between postoperative changes in reward-related feeding behavior and surgery type (Supplementary Table 7), none of these variables remained significant after testing in each surgical group separately (Supplementary Table 8). Change in a different set of variables was associated with %WL at the end of follow-up in the control group (Supplementary Table 9).
Confirmatory analyses of weight loss prediction in a different bariatric cohort
After identification of sweet intensity as a predictor of %WL 11 to 18 months following bariatric surgery, we explored another bariatric surgery cohort to address the replicability of this finding. In a post–bariatric surgery cohort recruited for another study, assessments for reward-related psychometric and gustatory measures, including sweet intensity perception, were performed approximately 2.5 years after surgery (Supplementary Tables 10 and 11). In 16 patients (10 post-GB and 6 post-SG), weight was reassessed, on average, 4 years after baseline, revealing nonsignificant differences of %WL between the 2 groups [post-GB, −13.9% (9.9%) vs. post-SG, −6.3% (6.4%), respectively; P = 0.1]. Across all patients, there was a significant correlation between sweet intensity at baseline assessment and %WL (r = 0.5; P = 0.04; Figure 3A). While this correlation did not remain significant when groups were analyzed separately, the overall trend seemed to be determined primarily by the post-GB group (r = 0.5; P = 0.1) rather than the post-SG group (r = −0.02; P = 0.967; Figure 3A). Additionally, weight change was associated with hedonic hunger, overall (PFS – Food tasted: r = −0.6; P = 0.02) and in the post-GB group (r = −0.9; P = 0.001), but not the post-SG group (r = −0.4; P = 0.5), as well as with acceptance for alcohol (FARS – Alcohol, r = −0.5; P = 0.03). The same study also recruited a group of healthy volunteers, 48 of whom were reassessed after an average 5.6 years of follow-up, revealing a %WL of −2.5% (6.8%). However, among 40 participants with full gustatory assessments at baseline, weight changes did not correlate with baseline sweet intensity ratings (r = −0.1; P = 0.7; Figure 3C), but rather with bitter intensity (r = 0.3; P = 0.04; Figure 3D), in a correlation that was absent in the surgical group (r = 0.01; P = 0.962; Figure 3B; Supplementary Table 12). The correlations observed in both the post–bariatric surgery and healthy volunteer groups were conserved when analyses were restricted to participants for whom the follow-up assessment of weight was measured in the lab, rather than being self-reported or obtained from clinical records (see Supplementary Material for details).
FIGURE 3.
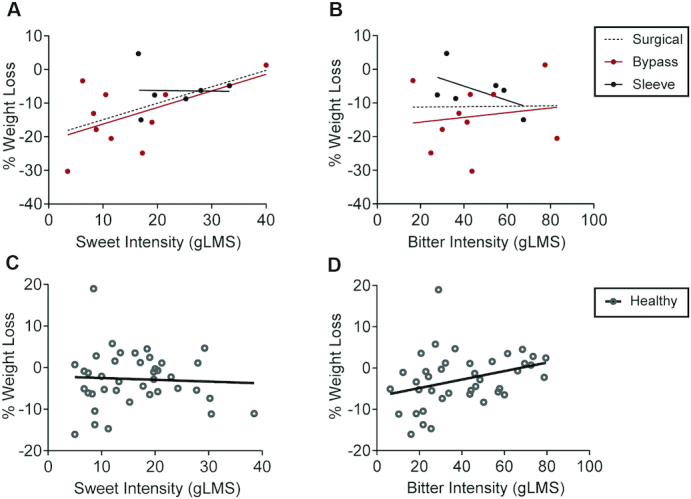
Associations between sweet intensity perception and %WL in a long-term follow-up bariatric cohort and comparison with healthy volunteers. (A) Correlations between sweet intensity perception and weight loss in the post–bariatric surgery cohort. The correlations between sweet intensity and %WL 4 years after the study baseline (2.5 years after surgery) were r = 0.5 (P = 0.04; n = 16) for the post–bariatric surgery group, r = 0.5 (P = 0.1; n = 10) for the GB group, and r = −0.02 (P = 1.0; n = 6) for the SG group. (B) Correlations between bitter intensity perception and weight loss in the post–bariatric surgery cohort. The correlations between bitter intensity and %WL 4 years after the study baseline (2.5 years after surgery) were r = 0.01 (P = 1.0; n = 16) for the post–bariatric surgery group, r = 0.2 (P = 0.7; n = 10) for the GB group, and r = −0.5 (P = 0.3; n = 6) for the SG group. (C) Correlation between sweet intensity perception and weight loss in healthy volunteers. The correlation between sweet intensity and %WL 5.6 years after the study baseline was r = −0.1 (P = 0.7; n = 40). (D) Correlation between bitter intensity perception and weight loss in healthy volunteers. The correlation between bitter intensity and %WL 5.6 years after the study baseline was r = 0.3 (P = 0.04; n = 40). Pearson correlation (r) was used to assess correlations between gustatory measures and %WL in healthy and surgical groups. %WL, percentage of weight loss; GB, gastric bypass; gLMS, general labeled magnitude scale; SG, sleeve gastrectomy.
Discussion
We tested whether gustatory and psychometric measures of reward sensitivity are predictors of weight loss following bariatric surgery, while exploring their postoperative changes. To address limitations of previous studies (16–18, 20–22), preliminary analyses were conducted to compare surgical patients and others receiving conservative treatment, using larger sample sizes and longer follow-ups. Bariatric surgery resulted in greater %WL, as well as a global change of feeding behavior, evidenced by significant reductions in scores for reward-related feeding behavior, feeding behavior traits, and acceptance of several foods, among which only hedonic hunger was a predictor of post-surgery %WL. Gustatory measures—namely, ratings of sweet intensity and pleasantness—did not differ between surgical patients and controls. However, baseline ratings of sweet intensity predicted postsurgery %WL and, contrary to other variables, including hedonic hunger, postoperative change in this variable was also associated with %WL. Associations of sweet intensity ratings with weight change were confirmed in a distinct post–bariatric surgery cohort and were absent in patients on the waitlist for surgery, as well as in healthy subjects. Thus, the surgically induced readjustment of the weight set point (8) seems to be associated with sweet taste.
Sweet intensity ratings in surgical patients reflected the most consistent finding of this study. At a group-level comparison with controls, gustatory measures were not significantly altered by surgery. However, preoperative sweet intensity predicted %WL at 11 to 18 months postsurgery across both surgery types. Furthermore, patients with greater %WL were also those in whom sweet intensity, as well as sweet food acceptance, was most decreased relative to baseline. In the control group, however, sweet intensity ratings were not associated with weight loss, possibly as a result of insufficient statistical power, less weight loss, or differences in weight loss mechanisms. These findings are in accordance with those reported by Nielsen et al. where, despite the absence of a specific effect of GB or SG on food preferences measured directly in a buffet, patients that shifted food preferences were also those that lost more weight, reinforcing the possibility that variable modulation of the physiological mechanisms that influence food reward may underlie heterogeneity in response to surgery (41). Our results also expand on previous work showing an interaction between presurgical sucrose wanting ratings—but not aspartame wanting, sucrose liking, or aspartame liking—and bariatric surgery type (GB compared with SG) on predictions of weight loss (23). Another recent study has shown that higher presurgical liking ratings for sugar-sweetened milk predicted greater weight loss 6 months after GB (15). We found that sucrose intensity ratings reflect susceptibility to weight loss across surgery types, rather than only in a specific surgery type, possibly because we obtained a pure measure of sweet taste reactivity rather than responses to ingested sucrose or the taste of sweetened milk, had a longer time of follow-up, and, importantly, did not observe differences in weight outcomes between surgical groups.
In a post–bariatric surgery cohort, with an assessment approximately 2.5 years after surgery, sweet intensity was also associated with %WL 4 years later, when most patients were experiencing weight gain relative to baseline. This provides additional evidence supporting the relationship between sweet intensity perception and post–bariatric surgery weight regulation, suggesting it persists after the active phase of weight loss. Importantly, in a healthy volunteer cohort, weight change was associated with baseline bitter intensity rather than sweet intensity, suggesting the latter is specific for bariatric surgery and raising hypotheses for future research.
Regarding psychometric measures of reward-related feeding behavior, only preoperative hedonic hunger was predictive of weight loss, but with an inverse relationship with %WL. Moreover, a postsurgical change of hedonic hunger was not associated with %WL, further suggesting that hedonic hunger is not redundant with sweet intensity measures. Food addiction and feeding behavior traits, while decreasing after surgery relative to the control group, did not predict postsurgery weight loss, in accordance with findings for food-related disinhibition (6) and both external and emotional eating (42). Hedonic hunger may be predictive of bariatric outcomes, because the PFS is not a measure of consumption per se, but instead captures anticipatory reward (27, 43), distinguishing it from feeding behavior traits that may merely reflect obesity status.
Analyses conducted to assess predictions of %WL according to surgery type showed that several psychometric measures of reward-related feeding behavior—specifically, hedonic hunger (PFS – Food tasted), addiction-like feeding behavior, and externally driven eating—predicted poorer %WL in GB patients but not SG patients. In the SG group, the effect sizes for each %WL predictor (β coefficients) were not significant and were positive rather than negative, suggesting that this did not result merely from a lack of statistical power. In the post–bariatric surgery cohort, with longer follow-ups, the Food tasted PFS subscale was the only parameter where GB-specific associations with weight change were consistent, suggesting that the vulnerability to the level of proximity and taste of food may be a specific determinant of weight loss for these patients. The association between %WL and preoperative alcohol acceptance in GB patients was not consistent across cohorts, possibly due to the exclusion of patients with alcohol use disorders in the post–bariatric surgery cohort. Nevertheless, others have shown that GB is independently related to increased odds of alcohol use disorders (44), supporting the need to monitor alcohol consumption.
This study does not provide mechanistic insight regarding the associations between sweet taste and post–bariatric surgery weight loss. The involvement of striatal dopamine seems plausible, since sucrose preference is inversely correlated with dopamine type 2 receptor binding in nonobese subjects but not obese subjects (45). It is possible that associations between sucrose preferences and dopamine type 2 receptor binding, which are lost among patients with obesity (45), may be recovered after bariatric surgery. Also, among patients with obesity, μ-opioid signaling may be related to sensitivity to reward, given evidence for decreased μ-opioid receptors in severe obesity (46), reverting in some patients 6 months after GB or SG (47). Interestingly, in patients with alcohol dependence, a sweet-liking phenotype was associated with a positive response to treatment with naltrexone, an opioid receptor antagonist (48). Finally, changes in neural and gustatory systems are likely to be in close interaction with hormonal modifications (9), which may be reflected by sweet sensitivity.
Our results should, nevertheless, be interpreted considering the study design. For instance, patients were not randomized to different treatment groups. This was attenuated for control versus surgical group comparisons, because the 2 had the same treatment indication although they were at different phases of the process, while surgery type differences may be diluted by the multicentric nature of the study. Importantly, at baseline, significant differences between the treatment groups were minor and, after surgery, there were no significant differences in weight outcomes. Furthermore, our major findings reflect predictions of the effects of surgery within specific groups, rather than comparisons between treatment groups. Regarding control versus surgical group comparisons, the complexity of approval for surgery conditioned a single follow-up assessment in the control group, which was at a slightly different time point when compared with the surgical group. However, since the majority of weight loss is achieved early after surgery and our analysis strategy considers time of assessment, this is unlikely to have determined the expressive prospective differences observed between groups.
Conclusions
We showed, for the first time, that preoperative sweet intensity predicts weight loss after surgery and that postsurgery changes in this variable track the amount of weight loss in each patient. While taste-related measures will require further clinical validation, their integration with other clinical and demographic variables may allow for the improved characterization of favorable phenotypes to bariatric surgery–induced weight loss.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—AJO-M: designed the research; GR and AJO-M: wrote the paper and had primary responsibility for final content; and all authors: conducted research, provided essential reagents and materials, analyzed data, and read and approved the final manuscript.
Author disclosures: AJO-M is a recipient of a grant from Schuhfried GmBH for norming and validation of cognitive tests; is national coordinator for Portugal of a non-interventional study (EDMS-ERI-143085581, 4.0) to characterize a treatment-resistant depression cohort in Europe, sponsored by Janssen-Cilag Ltd; and is national coordinator of a trial of psilocybin therapy for treatment-resistant depression, sponsored by Compass Pathways, Ltd (EudraCT NUMBER: 2017–003288–36). All other authors have no conflicts of interest to declare.
Notes
The study was supported by grants from the BIAL Foundation (176/10) and from Fundação para a Ciência e Tecnologia through a Junior Research and Career Development Award from the Harvard Medical Portugal Program (HMSP/ICJ/0020/2011) and grant PTDC/MED-NEU/31331/2017 to AJO-M. GR was funded by doctoral fellowships from Universidade de Lisboa (BD/2015Call) and Fundação para a Ciência e Tecnologia (FCT; SFRH/BD/128783/2017). ABF was funded by a postdoctoral fellowship from FCT (SFRH/BPD/880972/2012). GC was funded by a doctoral fellowship from FCT (SFRH/BD/130210/2017). ST was funded through the Center for Psychology at University of Porto (FCT UIDP/00050/2020).
The funding sources did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation or review of the manuscript.
Supplementary Tables 1–12 and Supplementary Material are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: %WL, percentage of weight loss; DEBQ, Dutch Eating Behavior Questionnaire; FARS, Food Action Rating Scale; GB, gastric bypass; LM-ELM, linear mixed-effects longitudinal models; MLR, multiple linear regression; PFS, Power of Food Scale; SG, sleeve gastrectomy; T2DM, type 2 diabetes mellitus; YFAS, Yale Food Addiction Scale.
Contributor Information
Gabriela Ribeiro, Champalimaud Research & Clinical Centre, Champalimaud Centre for the Unknown, Lisboa, Portugal; Lisbon Academic Medical Centre PhD Program, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal.
Marta Camacho, Champalimaud Research & Clinical Centre, Champalimaud Centre for the Unknown, Lisboa, Portugal.
Ana B Fernandes, Champalimaud Research & Clinical Centre, Champalimaud Centre for the Unknown, Lisboa, Portugal; NOVA Medical School, NMS, Universidade Nova de Lisboa, Lisboa, Portugal.
Gonçalo Cotovio, Champalimaud Research & Clinical Centre, Champalimaud Centre for the Unknown, Lisboa, Portugal; NOVA Medical School, NMS, Universidade Nova de Lisboa, Lisboa, Portugal; Department of Psychiatry and Mental Health, Centro Hospitalar de Lisboa Ocidental, Lisboa, Portugal.
Sandra Torres, Faculdade de Psicologia e de Ciências da Educação, Universidade do Porto, Porto, Portugal; Centro de Psicologia da Universidade do Porto, Porto, Portugal.
Albino J Oliveira-Maia, Champalimaud Research & Clinical Centre, Champalimaud Centre for the Unknown, Lisboa, Portugal; NOVA Medical School, NMS, Universidade Nova de Lisboa, Lisboa, Portugal; Department of Psychiatry and Mental Health, Centro Hospitalar de Lisboa Ocidental, Lisboa, Portugal.
Data Availability
According to the International Committee of Medical Journal Editors (ICMJE), data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149(3):275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, Schultes B, Beglinger C, Drewe J, Schiesser Met al. . Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: The SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jakobsen GS, Småstuen MC, Sandbu R, Nordstrand N, Hofsø D, Lindberg M, Hertel JK, Hjelmesæth J. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA. 2018;319(3):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA. 2018;320(15):1560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell JE, Christian NJ, Flum DR, Pomp A, Pories WJ, Wolfe BM, Courcoulas AP, Belle SH. Postoperative behavioral variables and weight change 3 years after bariatric surgery. JAMA Surg. 2016;151(8):752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Konttinen H, Peltonen M, Sjöström L, Carlsson L, Karlsson J. Psychological aspects of eating behavior as predictors of 10-y weight changes after surgical and conventional treatment of severe obesity: Results from the Swedish Obese Subjects intervention study. Am J Clin Nutr. 2015;101(1):16–24. [DOI] [PubMed] [Google Scholar]
- 7. Sharples AJ, Mahawar K, Cheruvu CV. Systematic review and retrospective validation of prediction models for weight loss after bariatric surgery. Surg Obes Relat Dis. 2017;13(11):1914–20. [DOI] [PubMed] [Google Scholar]
- 8. Al-Najim W, Docherty NG, le Roux CW. Food intake and eating behavior after bariatric surgery. Physiol Rev. 2018;98(3):1113–41. [DOI] [PubMed] [Google Scholar]
- 9. Nance K, Acevedo MB, Pepino MY. Changes in taste function and ingestive behavior following bariatric surgery. Appetite. 2020;146:104423. doi:10.1016/j.appet.2019.104423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miras AD, Jackson RN, Jackson SN, Goldstone AP, Torsten O, Hackenberg T, Spector AC, le Roux CW. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am J Clin Nutr. 2012;96(3):467–73. [DOI] [PubMed] [Google Scholar]
- 11. Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr. 2010;92(2):277–83. [DOI] [PubMed] [Google Scholar]
- 12. Ullrich J, Ernst B, Wilms B, Thurnheer M, Schultes B. Roux-en-Y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obes Surg. 2013;23(1):50–5. [DOI] [PubMed] [Google Scholar]
- 13. Cushing CC, Benoit SC, Peugh JL, Reiter-Purtill J, Inge TH, Zeller MH. Longitudinal trends in hedonic hunger after Roux-en-Y gastric bypass in adolescents. Surg Obes Relat Dis. 2014;10(1):125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivezaj V, Wiedemann A, Grilo C. Food addiction and bariatric surgery: A systematic review of the literature. Obes Rev. 2017;18(12):1386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith KR, Papantoni A, Veldhuizen MG, Kamath V, Harris C, Moran TH, Carnell S, Steele KE. Taste-related reward is associated with weight loss following bariatric surgery. J Clin Invest. 2020;130(8):4370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nance K, Eagon JC, Klein S, Pepino MY. Effects of sleeve gastrectomy vs. Roux-en-Y gastric bypass on eating behavior and sweet taste perception in subjects with obesity. Nutrients. 2017;10(1):18. doi:10.3390/nu10010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altun H, Hanci D, Altun H, Batman B, Serin RK, Karip AB, Akyuz U. Improved gustatory sensitivity in morbidly obese patients after laparoscopic sleeve gastrectomy. Ann Otol Rhinol Laryngol. 2016;125(7):536–40. [DOI] [PubMed] [Google Scholar]
- 18. Holinski F, Menenakos C, Haber G, Olze H, Ordemann J. Olfactory and gustatory function after bariatric surgery. Obes Surg. 2015;25(12):2314–20. [DOI] [PubMed] [Google Scholar]
- 19. Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, le Roux CW. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104(5):709–21. [DOI] [PubMed] [Google Scholar]
- 20. Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S. Changes in taste perception and eating behavior after bariatric surgery–induced weight loss in women. Obesity. 2014;22(5):E13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients’ taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc. 1995;95(6):666–70. [DOI] [PubMed] [Google Scholar]
- 22. Scruggs DM, Buffington C, Cowan GS. Taste acuity of the morbidly obese before and after gastric bypass surgery. Obes Surg. 1994;4(1):24–8. [DOI] [PubMed] [Google Scholar]
- 23. Perez-Leighton CE, Hamm JD, Shechter A, Tamura S, Laferrère B, Pi-Sunyer X, Albu J, Greenberg D, Kissileff HR. Preoperative liking and wanting for sweet beverages as predictors of body weight loss after Roux-en-Y gastric bypass and sleeve gastrectomy. Int J Obes (Lond). 2020;44(6):1350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murray SM, Tweardy S, Geliebter A, Avena NM. A longitudinal preliminary study of addiction-like responses to food and alcohol consumption among individuals undergoing weight loss surgery. Obes Surg. 2019;29(8):2700–3. [DOI] [PubMed] [Google Scholar]
- 25. Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, Ko CY, Gibbons MM. Preoperative predictors of weight loss following bariatric surgery: Systematic review. Obes Surg. 2012;22(1):70–89. [DOI] [PubMed] [Google Scholar]
- 26. Hatoum IJ, Kaplan LM.. Advantages of percent weight loss as a method of reporting weight loss after Roux‐en‐Y gastric bypass. Obesity. 2013;21(8):1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Ochner CN, Coletta MC, Bellace D, Wallaert Met al. . The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53(1):114–18. [DOI] [PubMed] [Google Scholar]
- 28. Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Karlsson J, Lowe MR. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: Development and measurement properties. Int J Obes. 2009;33(8):913–22. [DOI] [PubMed] [Google Scholar]
- 29. Ribeiro G, Santos O, Camacho M, Torres S, Mucha-Vieira F, Sampaio D, Oliveira Maia AJ. Translation, cultural adaptation and validation of the Power of Food Scale for use by adult populations in Portugal. Acta Med Port. 2015;28(5):575–82. [DOI] [PubMed] [Google Scholar]
- 30. Torres S, Camacho M, Costa P, Ribeiro G, Santos O, Vieira FM, Brandão I, Sampaio D, Oliveira-Maia AJ. Psychometric properties of the Portuguese version of the Yale Food Addiction Scale. Eat Weight Disord. 2017;22(2):259–67. [DOI] [PubMed] [Google Scholar]
- 31. Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52(2):430–6. [DOI] [PubMed] [Google Scholar]
- 32. Viana V, Sinde S. Estilo alimentar: Adaptação e validação do questionário holandês do comportamento alimentar (Eating style: A validation study of the Dutch Eating Behavior Questionnaire for the Portugese population). Psicologia: Teoria, Investigação e Prática. 2003;8(1–2):59–71. [Google Scholar]
- 33. Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5(2):295–315. [Google Scholar]
- 34. Schutz HG. Food Action Rating Scale for measuring food acceptance. J Food Science. 1965;30(2):365–74. [Google Scholar]
- 35. Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. [Database record]. APA PsycTests;. 1996. doi: 10.1037/t00742-000. [DOI] [Google Scholar]
- 36. Landis BN, Welge-Luessen A, Brämerson A, Bende M, Mueller CA, Nordin S, Hummel T. “Taste Strips”–A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol. 2009;256(2):242–8. [DOI] [PubMed] [Google Scholar]
- 37. Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the “Labeled Magnitude Scale” for measuring sensations of taste and smell. Chem Senses. 1996;21(3):323–34. [DOI] [PubMed] [Google Scholar]
- 38. Lim J, Wood A, Green BG. Derivation and evaluation of a labeled hedonic scale. Chem Senses. 2009;34(9):739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fons M, Osterhammel PA. Electrogustometry. Arch Otolaryngol Head Neck Surg. 1966;83(6):538–42. [DOI] [PubMed] [Google Scholar]
- 40. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 41. Søndergaard Nielsen M, Rasmussen S, Just Christensen B, Ritz C, le Roux CW, Berg Schmidt J, Sjödin A. Bariatric surgery does not affect food preferences, but individual changes in food preferences may predict weight loss. Obesity. 2018;26(12):1879–87. [DOI] [PubMed] [Google Scholar]
- 42. Holsen LM, Davidson P, Cerit H, Hye T, Moondra P, Haimovivi F, Sogg S, Shikora S, Goldstein JM, Evins AEet al. . Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int J Obes. 2018;42(4):785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ribeiro G, Camacho M, Santos O, Pontes C, Torres S, Oliveira-Maia AJ. Association between hedonic hunger and body-mass index versus obesity status. Sci Rep. 2018;8(1):5857. doi:10.1038/s41598-018-23988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. King WC, Chen J-Y, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, Courcoulas AP, Pories WJ, Yanovski SZ. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pepino MY, Eisenstein SA, Bischoff AN, Klein S, Moerlein SM, Perlmutter JS, Black KJ, Hershey T. Sweet dopamine: Sucrose preferences relate differentially to striatal D2 receptor binding and age in obesity. Diabetes. 2016;65(9):2618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, Salminen P, Nuutila P, Nummenmaa L. Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35(9):3959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karlsson H, Tuulari J, Tuominen L, Hirvonen J, Honka H, Parkkola R, Helin S, Salminen P, Nuutila P, Nummenmaa L. Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Mol Psychiatry. 2016;21(8):1057–62. [DOI] [PubMed] [Google Scholar]
- 48. Garbutt JC, Kampov-Polevoy AB, Kalka-Juhl LS, Gallop RJ. Association of the sweet-liking phenotype and craving for alcohol with the response to naltrexone treatment in alcohol dependence: A randomized clinical trial. JAMA Psych. 2016;73(10):1056–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
According to the International Committee of Medical Journal Editors (ICMJE), data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.