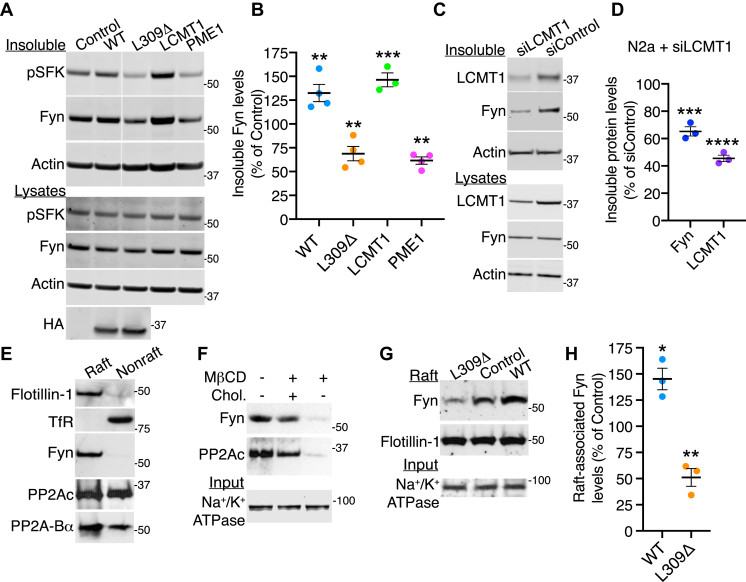
Figure 2.
Changes in PP2A methylation influence the discrete membrane distribution of Fyn in N2a cells.A, representative immunoblots of pY416-SFK (pSFK), Fyn, and actin in total lysates and NP-40 detergent–insoluble fractions prepared from N2a cells stably expressing WT PP2Ac, the L309Δ PP2Ac mutant, LCMT1, PME1, or empty vector (control). Panels in detergent-insoluble fractions originated from the same blot. B, quantification of Fyn levels in NP-40 detergent–insoluble fractions from these cells. Data (mean ± SEM from n = 3–4 independent experiments) were appraised using one-way ANOVA (F (4, 14) = 33.44; p < 0.0001) with Dunnett’s post hoc test. ∗∗p < 0.01, ∗∗∗p < 0.001, versus control. C, total lysates and NP-40 detergent–insoluble fractions purified from N2a cells transfected with a validated siRNA targeted to LCMT1 (siLCMT1) or a mismatch siRNA control (siControl) were analyzed by Western blotting for the presence of Fyn and LCMT1. D, Fyn and LCMT1 protein levels were decreased in detergent-insoluble fractions from siLCMT1 relative to siControl-transfected N2a cells. Data (mean ± SEM; n = 3 separate experiments) were analyzed using a student t-test. ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. E, representative immunoblots of PP2ABα and PP2Ac subunits, Fyn, flotillin-1, and transferrin receptor (TfR) in raft and nonraft membrane fractions purified from N2a cells. Similar results were obtained in three separate purifications. F, representative distribution of PP2Ac and Fyn in aliquots (15 μg) of raft fractions purified from N2a cells that were incubated for 15 min in a serum-deficient medium in the absence (−) or presence (+) of the cholesterol depletion agent MβCD or cholesterol (Chol). Total membrane fractions (input) from these cells were probed with an antibody against the membrane marker, sodium potassium adenosine triphosphatase (Na+/K+ ATPase). G, representative Western blot analysis of total membrane fractions (input) and rafts purified from EV-, WT-, or L309Δ-transfected N2a cells. H, relative levels of raft-associated Fyn were quantified in EV-, WT-, and L309Δ-expressing N2a cells. Data (mean ± SEM from n = 3 separate purifications) were analyzed using a student t-test. ∗p < 0.05; ∗∗p < 0.01, versus control. EV, empty vector; LCMT1, leucine carboxyl methyltransferase 1; N2a, Neuro-2a; MβCD, methyl-β-cyclodextrin; PP2A, PP2A, protein phosphatase 2A; PP2Ac, catalytic “C” subunit of PP2A.