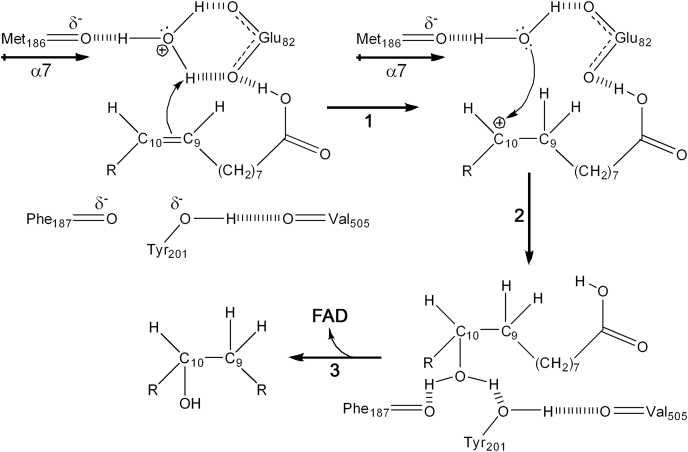
Figure 9.
Acid-catalyzed alkene hydration by OhyA. FAD binding leaves a buried water in the active site where the α7 dipole, Glu82 and the oleate carboxyl create an environment that stabilizes the hydronium ion (H3O+). The acidic water proton (H+) is attacked by the oleate π bond to form a planar carbocation as a transient intermediate (1). Water then attacks the carbocation to form an oxonium water adduct that is stabilized by the hydrogen bond network involving Tyr201, Phe187 and Val505 (2). The active site lid opens, FAD leaves and the proton is released to the hydrated active site leading to product formation (3). FAD, flavin adenine dinucleotide; OhyA, oleate hydratase; PEG400, polyethylene glycol 400.