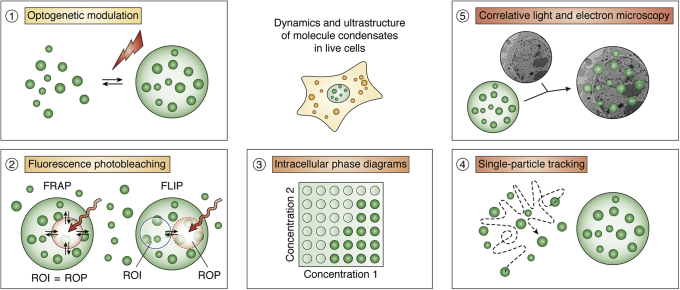
Figure 2.
Observing and quantifying the dynamics and ultrastructure of condensates in live cells. Counterclockwise from top left: 1. Optogenetic manipulation of condensates dissociates condensation of specific cellular components from cell-wide effects of environmental perturbations. Condensation can be modulated by activating light-sensitive protein-interaction domains using specific wavelengths (126, 127, 128). These methods are powerful as they can modulate phase behavior of specific components without otherwise perturbing the cell; they alter material properties of condensates rapidly, dissect the contributions of individual interactions without being constrained by the cellular milieu; and enable studies of the biochemical impact of rapidly altered molecular clustering in the cell (41, 126, 129, 130). 2. In fluorescence recovery after photobleaching (FRAP), a small region is photobleached and the rate of the recovery of fluorescence in this region serves as a readout of the effect of the local environment on the molecule of interest. For this reason, FRAP has been widely used to assess liquid-like properties (131, 132). ROI, region of interest; ROP, region of photobleaching. Fluorescence loss in photobleaching (FLIP) is similar to FRAP but is used to investigate the exchange of material between condensates. In FLIP, a small region is repeatedly photobleached and the loss of fluorescence is measured in another region (131). 3. Studying phase separation from purified components in the test tube allows precise and systematic investigation of the effects of composition, temperature, pH, salt, etc., to build a phase diagram (133, 134, 135). While such manipulations are less easily achieved in cells, several recent studies have reported strategies to obtain phase transition information from intracellular fluorescence measurements (42, 43, 49, 78). 4. Single particle tracking (SPT) is a powerful tool to study dynamic recruitment of molecules to condensates (45, 65, 136). 5. CLEM, correlative light and electron microscopy is an emerging tool that holds great potential to uncover ultrastructural details of condensates. It involves two steps. In the first step, a fluorescence-tagged moiety is detected to extract spatial information with biomolecular specificity. In the second step, the same sample is then imaged using electron microscopy. Correlating features in the electron micrograph with the fluorescence signal can identify specific components within the ultrastructure of liquid-like condensates, which often is more challenging to achieve via electron microscopy alone (137, 138).