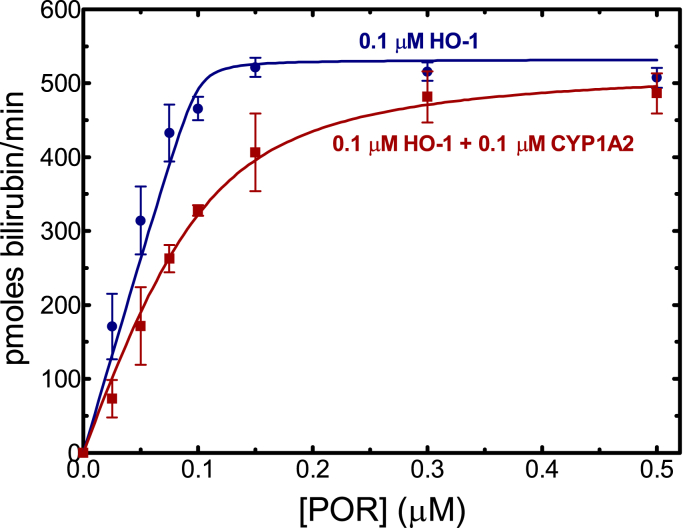
Figure 7.
Effect of CYP1A2 on heme degradation by HO-1. HO-1 (0.1 μM) was reconstituted in DLPC at POR concentrations ranging from 0.025 to 0.5 μM in the absence (green) and presence (brown) of 0.1 μM CYP1A2. Heme oxygenase activity was determined by monitoring the formation of bilirubin using a coupled assay containing biliverdin reductase. The presence of CYP1A2 led to inhibition of HO-1 activity at subsaturating POR but not at higher POR saturation. Data were fit to the Morrison tight binding equation using nonlinear regression. Plotted data represent the mean and SD of 4 determinations. DLPC, L-α-dilauroyl-sn-glycero-3-phosphocholine; HO-1, heme oxygenase 1; POR, NADPH-cytochrome P450 reductase.