Figure 2.
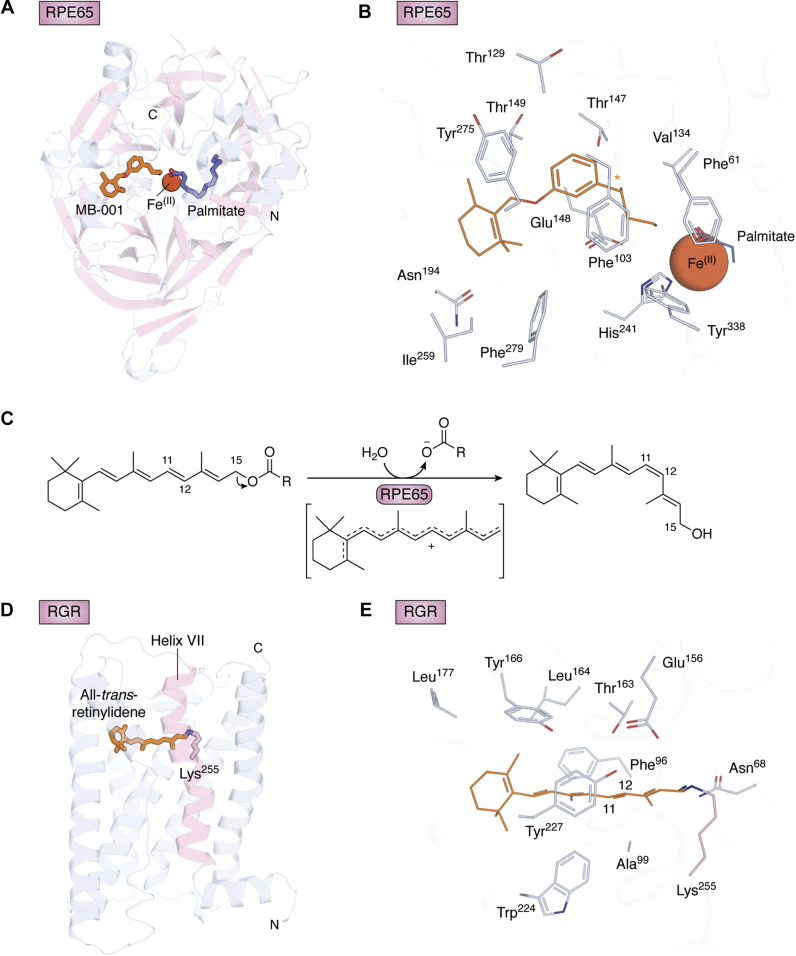
Three-dimensional structural models of visual cycle retinoid isomerases.A, crystal structure of bovine RPE65 in complex with MB-001 (orange sticks), an 11-cis-retinoid mimetic, and the palmitate product of catalysis (purple sticks) (Protein Data Bank (PDB) accession code 4RSE). B, detailed view of the RPE65 active site showing residues in proximity to the retinoid-binding site. The orange asterisk indicates the predicted binding position of the retinoid C11-C12 bond. The iron ion is directly coordinated by a set of 4-His residues (only His241 is shown in the figure). Thr147, Phe103, Tyr338 play key roles in polyene isomerization stereoselectivity (54, 81, 82). C, RPE65-catalyzed isomerization reaction involving a putative carbocation intermediate. D, homology model of human RGR bound to all-trans-retinal via a Lys255 Schiff base. The model was generated using bovine rod opsin as a template (PDB accession code 3PXO) via the SWISS-MODEL server (183). Helix VII containing the chromophore-binding Lys255 residue is shown in pink. E, predicted model of the RGR active site. Residues that are absolutely conserved across a wide range of species from humans to zebrafish and within 4.5 Å of the retinylidene group (orange lines) are shown as lines. Aromatic residues Tyr166, Phe96, Tyr227, Trp224 could be critical in trans–cis stereospecificity, and Glu156 could be a counterion of the protonated Schiff base between retinal and Lys255.