Figure 3.
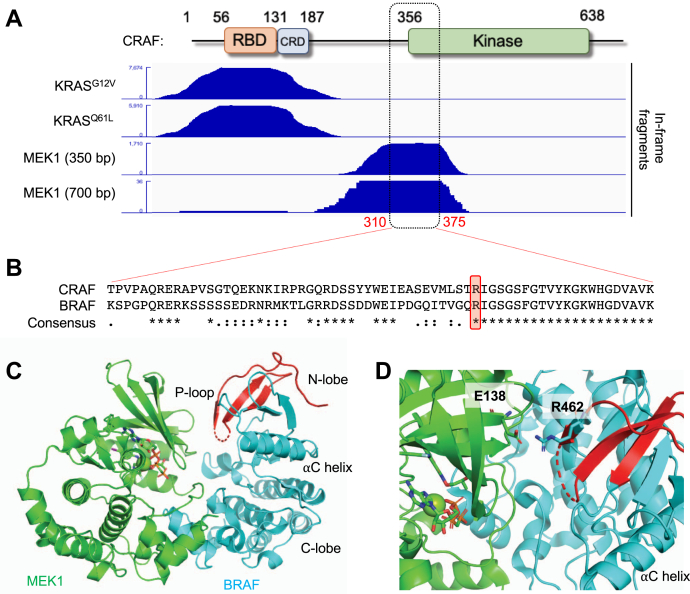
DoMY-Seq reveals the interaction interface between CRAF and MEK1.A, integrative Genomics Viewer (IGV) snapshots highlighting the CRAF-interacting domains of KRAS and MEK1 baits. The MEK1-binding motif is defined within aa 310 to 375. Note that shorter fragments (350 bp) provide better resolution than larger fragments (700 bp) in this assay. For each assay, two biological independent assays were performed. B, alignment between CRAF and BRAF shows a number of conserved amino acids present in the 310 to 375 motif. CRAF R354 and BRAF R462 are highlighted in red. C, overall structure of the BRAF (cyan) and MEK1 (green) interaction (PBD: 4MNE). Regulatory motifs of the BRAF kinase domain have been labeled. The aligned region of the CRAF MEK1 binding motif (310–375) identified by DoMY-Seq is highlighted in red and is present within the BRAF P-loop. D, a detailed snapshot of (C) reveals the presence of the key interacting residues R462 (BRAF) and E138 (MEK1). BRAF R462 is conserved in CRAF (R354). DoMY-Seq, Protein Domain mapping using Yeast 2 Hybrid-Next Generation Sequencing.