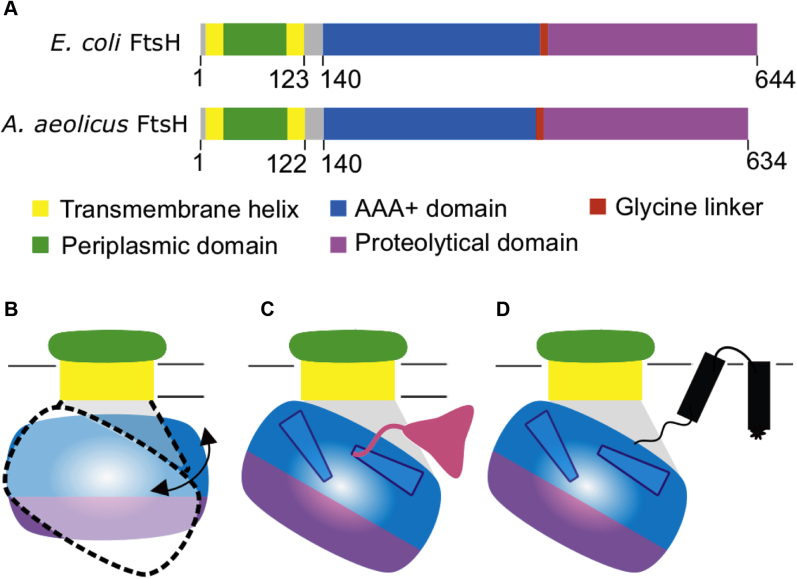
Figure 5.
Schematic representation of AaFtsH sequence and proposed model for substrate entry.A, bioinformatics tools and available structures of FtsH domains, from E. coli and A. aeolicus, show the presence of a loop-like peptide structure with ∼20 aa between membrane and AAA domains. The N-terminal periplasmic domain (green) is between two transmembrane helices (yellow). The second transmembrane helix is followed by a loop region (gray; see text), which is the link to the AAA+ domain (blue). Connected by the glycine linker (red), the C-terminal protease domain is shown in purple. B, a new model for substrate entry is proposed. The ∼20-aa flexible linker could allow the cytosolic domain of FtsH to tilt in relation to the membrane, creating a 30-Å wide gap that provides access of cytosolic (pink, C) and membrane protein substrates (black, D) to the central pore in a partially folded state.