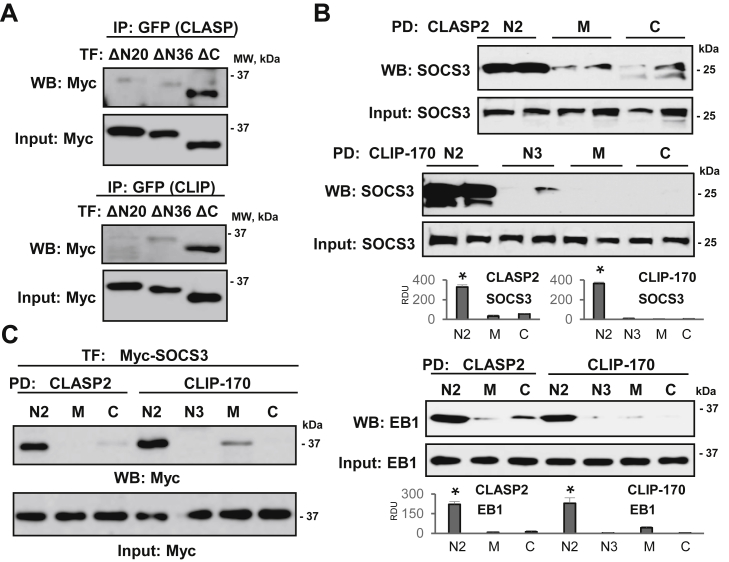
Figure 6.
N-terminal of SOCS3 is involved in its interaction with N2 domains of CLIP-170 and CLASP2. A, ECs with coexpression of Myc-tagged N-terminal deletion mutants of SOCS3: ΔN20, ΔN36, or C-terminal SOCS3 deletion mutant (ΔC47) lacking SOCS box and GFP-CLASP2 (Top); or GFP-CLIP-170 (Bottom) were used for co-IP assay with GFP antibody followed by membrane probing with Myc antibody. Equal volumes of total cell lysates were probed with Myc antibody as normalization control. B, pull-down (PD) assay of EC lysates was performed with purified GST-fusion proteins encoding N2, M, and C domains of CLASP2 or N2, N3, M, and C domains of CLIP-170 (Top). Endogenous SOCS3 from cell lysates bound to immobilized CLASP2 and CLIP-170 domains was detected by western blot with SOCS3 antibody. Probing total cell lysates with SOCS3 antibody was used as a normalization control. Pull-down assay of End-Binding Protein 1 (EB1) (Bottom) served as a positive control to confirm published EB1 interaction with CLASP2 and CLIP-170 domains. C, pull-down experiment was carried out as in (B) after overexpression of SOCS3 followed by immunoblotting with Myc antibody. Bar graphs depict the results of quantitative densitometry of western blot data; n = 4, ∗p < 0.05.