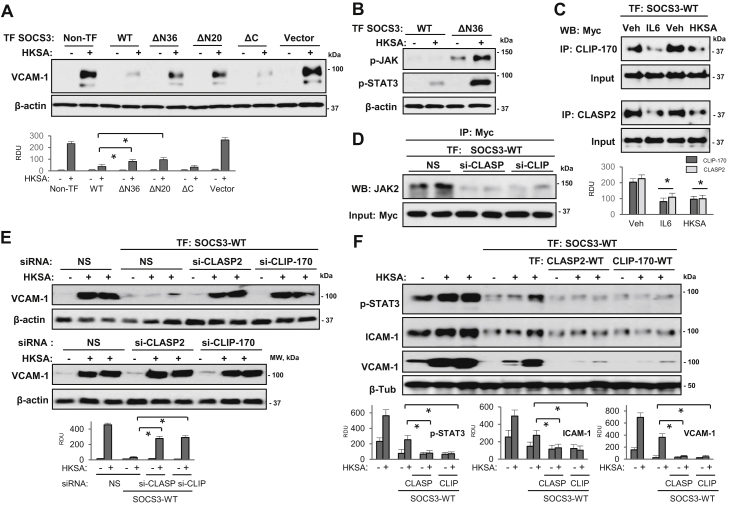
Figure 7.
N-terminal region of SOCS3 is essential for its inhibition of HKSA-induced inflammation. A, pulmonary ECs were transfected with WT SOCS3 and indicated mutants or empty vector followed by HKSA stimulation (5 × 108 particles/ml, 6 h) and western blot detection of VCAM-1 expression. Probing with β-actin was used as a normalization control. Bar graphs depict the results of quantitative densitometry of western blots; ∗p < 0.05, n = 5. B, phospho-JAK2 and phospho-STAT3 levels in HKSA-challenged EC with expression of Myc-SOCS3 or its Myc-ΔN36 mutant were detected by western blot. Probing with β-actin served as a normalization control. C, ECs with Myc-SOCS3 expression were exposed to IL-6 or HKSA (6 h), and co-IP with CLIP-170 and CLASP2 antibodies was followed by western blot with Myc antibody. D, ECs were cotransfected with nonspecific (NS), CLIP-170- or CLASP2-specific siRNAs and Myc-SOCS3 plasmid. Co-IP with Myc antibody was followed by western blot with JAK2 antibody. Probing of total cell lysates for Myc was used to verify equal inputs. E, ECs cotransfected with SOCS3-WT plasmid DNA and siRNAs targeting CLIP-170 or CLASP2 were treated with HKSA followed by western blot analysis of VCAM-1. Bar graph depicts the results of quantitative densitometry of western blots; n = 5, ∗p < 0.05. Lower panel: VCAM-1 expression in control and HKSA-stimulated EC transfected with nonspecific, CLIP-170-specific, or CLASP2-specific siRNAs; probing for β-actin served as a loading control. F, nontransfected pulmonary EC or cells with coexpression of SOCS3 and CLIP-170 or CLASP2 were stimulated with HKSA for indicated times. Phospho-STAT3, ICAM-1, and VCAM-1 expression in cell lysates was monitored by western blot. Bar graphs depict the results of quantitative densitometry of western blot data; n = 5, ∗p < 0.05. RDU, relative density units.