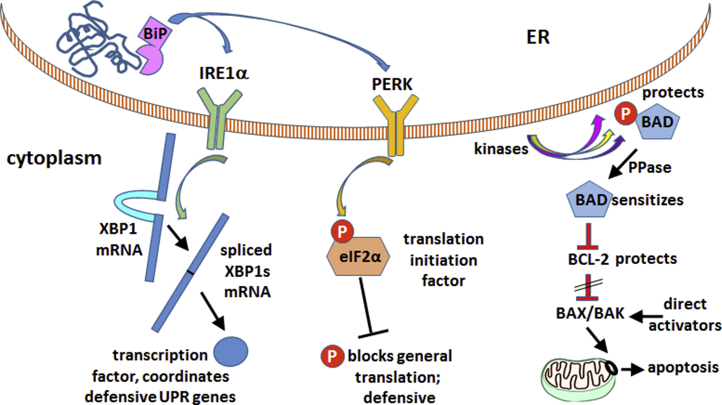
Figure 3.
Relevant pathways of the unfolded protein response (UPR). This diagram shows only a few of the components of the UPR but highlights three central elements that were examined here. The UPR begins with the recognition of luminal misfolded proteins by the chaperone BiP (GRP78), known to interact with both α and β Na,K-ATPase subunits during biosynthesis in Xenopus oocytes (22). The UPR initially activates defensive programs to expand the folding capacity of the ER. (Left) Activated IRE1α has a cytoplasmic nuclease activity needed to splice the XPB1 mRNA to XBP1s, changing its reading frame so that it encodes a master transcription factor for the defensive arm of the UPR. (Middle) Activated PERK phosphorylates an essential translation initiation factor, eIF2α. This attenuates translation, reducing the stress on the ER and making it possible for its resources to be redirected to defensive adaptations (37). (Right) If aggregated proteins nonetheless accumulate, the UPR activates apoptosis (38). The pathway utilizes the BCL-2 family proteins that regulate the formation of mitochondrial pores (black circle) by BAX and BAK to release cytochrome c (38). Current models hold that BAX and BAK are activated directly by members of one arm of the BCL-2 family (the direct activators), in response to various signals. Apoptosis is constitutively restrained, however, by BCL-2 itself, which binds and blocks BAX and BAK. For apoptosis to proceed, BCL-2 needs to be sequestered (crosshatch) by binding to BAD or other members of the sensitizer arm of the BCL-2 family. BAD binding to BCL-2 is attenuated by phosphorylation by a variety of prosurvival kinases (39, 40). Dephosphorylation of BAD by protein phosphatase (PPase), for example, by calcineurin, the Ca2+-activated phosphatase, will activate proapoptotic signaling (41). In sum, dephosphorylation of BAD, at Ser99 in this case, is an indication that BAD is free to inactivate BCL-2, making it more likely that BAX and BAK will respond to direct activators, i.e., sensitizing the cell to apoptosis.