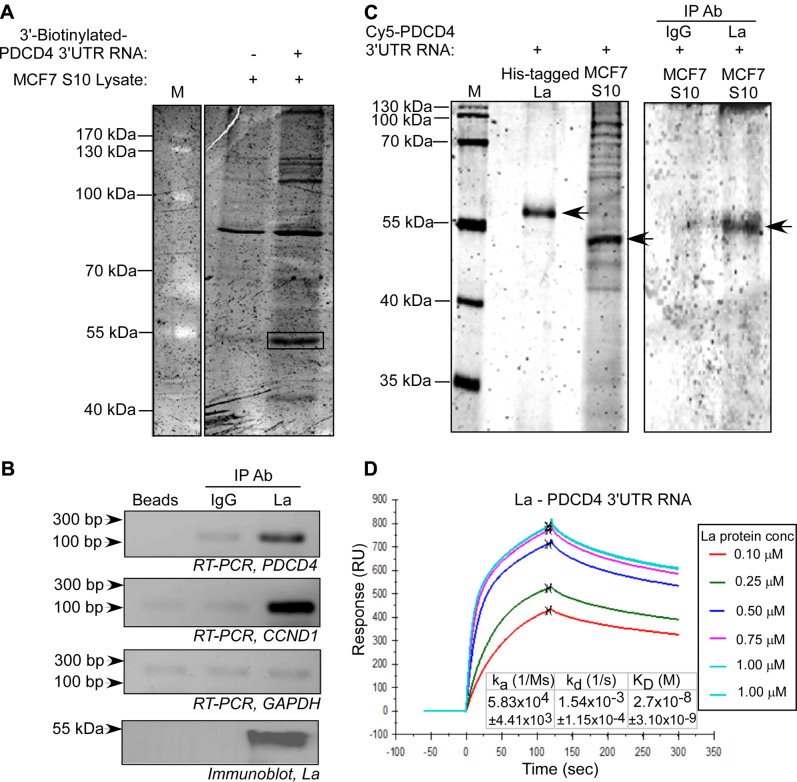
Figure 1.
RNA-binding protein La interacts with PDCD4 mRNA 3'UTR. A, five hundred micrograms MCF7 cytoplasmic lysate was incubated with 10 μg 3ʹ-biotinylated PDCD4 3ʹUTR RNA. Proteins associated with the RNA were purified by affinity chromatography and resolved by 10% SDS-PAGE. Protein band marked by black rectangle was found to contain La protein by mass spectrometry. Lane M contains protein molecular weight markers, second lane contains proteins eluted from streptavidin beads only, third lane contains proteins eluted from 3ʹ-biotinylated PDCD4 3ʹUTR RNA. B, cell lysates were immunoprecipitated with anti-La antibody or control IgG, and immunoprecipitated RNA was isolated and subjected to RT–PCR using PDCD4, CyclinD1 (CCND1), and GAPDH primers. RNA pulled down only with protein-A sepharose beads was analyzed for nonspecific interaction. The panel at the bottom represents immunoblot of La protein from the immunoprecipitate. C, Cy5-UTP-labeled PDCD4 3ʹUTR RNA was incubated either with purified, recombinant His-tagged La protein or MCF7 cytoplasmic lysate followed by UV crosslinking and RNase A digestion. The RNP complexes were resolved by 10% SDS-PAGE (left panel). The RNP complexes were immunoprecipitated with La antibody or IgG and resolved on 10% SDS-PAGE (right panel). The La RNP-complex bands are indicated by arrows. D, in vitro transcribed 3ʹ-biotinylated PDCD4 3ʹUTR RNA was immobilized on the Biacore SA chip. Increasing concentrations of purified La protein was flowed over the chip, and the Response Units were plotted against time. Binding constants (Ka, Kd, and KD) were calculated considering 1:1 binding kinetics. The binding constants represent the mean ± SEM from three independent experiments. La, lupus antigen; PDCD4, programmed cell death 4; 3ʹUTR, 3ʹ-untranslated region.