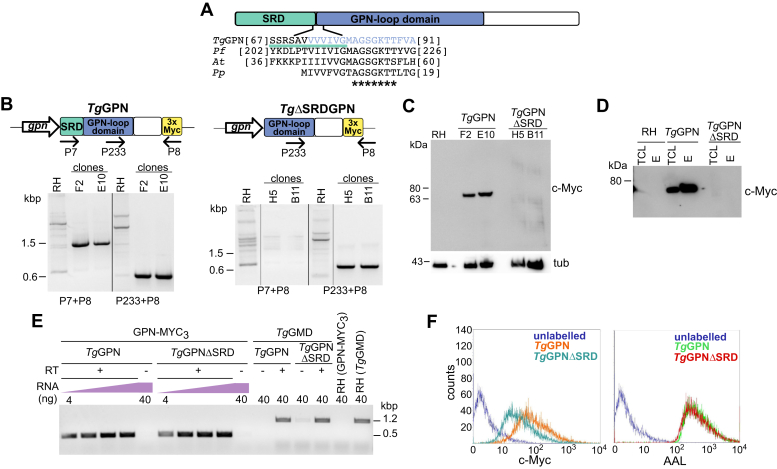
Figure 5.
O-fucosylated reporter proteins’ stability is affected by lack of SRD.A, schematic representation of the domains of TgGPN. Residues 1–79 were defined as SRD and TgGPNΔSRD starts at aa 80. The residues marked with asterisks are part of the active site of GPNs (59, 60). B, genotyping by PCR of the clones expressing either full-length or truncated GPN. All primers are listed in Table S1. Pf: Plasmodium falciparum, Pp: Pyrococcus abyssi. C, C-terminally MYC3-tagged TgGPN and TgGPNΔSRD were ectopically expressed in RH, and two pairs of clonal populations were analyzed by western blot with anti-cMYC. The mobility of full-length TgGPN-MYC3 suggested an apparent Mr higher than theoretical (46,600), possibly due to the O-fucosylation. No clear band was observed for TgGPNΔSRD. D, when AAL-enriched proteins were probed with anti-cMYC, full-length TgGPN was detected in both total cell lysate (TCL) and enriched fraction (E), whereas TgGPNΔSRD was not detected in either fraction. E, semiquantitative RT-PCR indicated comparable mRNA levels for full-length and truncated isoforms. T. gondii GDP-mannose 4,6-dehydratase (TgGMD) served as a positive control. F, flow cytometry showed a decrease in mAb 9E10 binding to TgGPNΔSRD-MYC3 compared with full-length TgGPN. Scale bars: 2 μm. Epi: epichromatin; tub: tubulin.