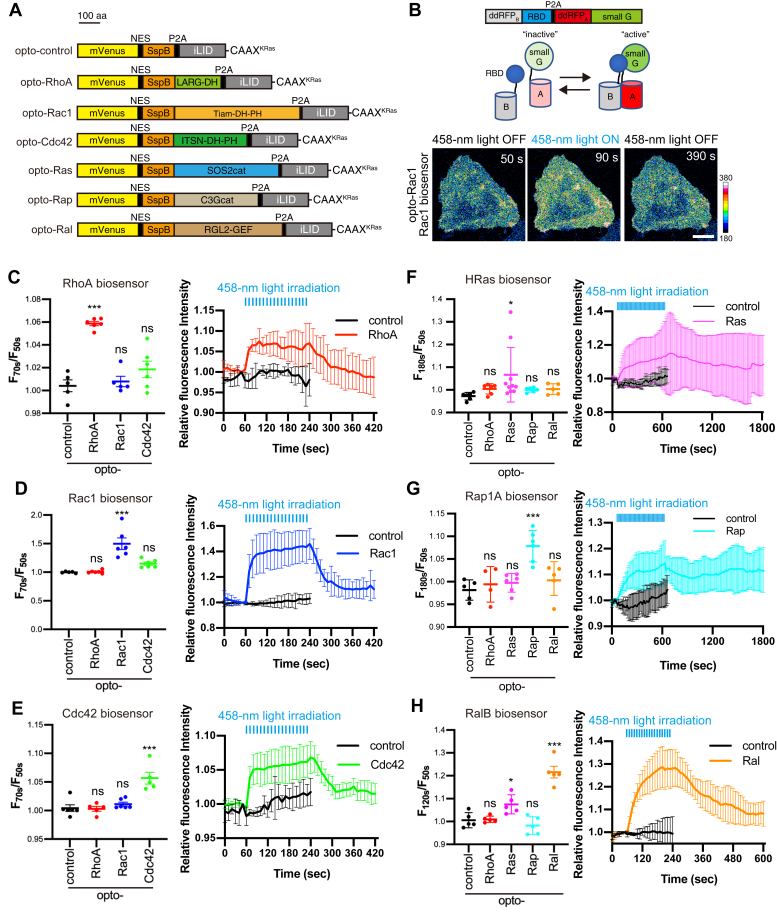
Figure 2.
Construction and characterization of optogenetic tools to control small GTPase activity.A, Schematic of opto-GTPases. B, Schematic of ddRFP-based small GTPase sensor (top and middle). Example images for the experiments (bottom). HeLa cells transiently expressing opto-Rac1 and Rac1 biosensors were irradiated using a 458-nm laser every 10 s during a period of 60–230 s. During irradiation, an increase in the fluorescence intensity of the Rac1 biosensor indicated an increase of Rac1 activity. The color bar indicates the range of Rac1 biosensor intensity. Scale bar = 20 μm. C–H, HeLa cells transiently expressing opto-GTPases and small GTPase biosensors were observed using confocal microscopy in Leibovitz’s L-15 medium. Opto-GTPases were activated by a multi-argon 458-nm laser every 10 s during a period of 60–230 s (C–E, H) or 60–350 s (F–G). Changes in the fluorescence intensity of biosensors before and after irradiation with the 458-nm light (left). Time course of small GTPase activity in cells expressing opto-control and opto-GTPase corresponding to each biosensor (right). Data are presented as the means ± SD. More than five cells were analyzed for each condition, and the actual number of cells is presented as the number of data points. Time course for all opto-GTPases is presented in Figure S3. Data were analyzed by one-way ANOVA followed by Dunnett’s test (C–E and G–H) or Brown–Forsythe ANOVA followed by Dunnett’s T3 test (F) between cells expressing opto-control and other groups. ANOVA F = 24.5 and p < 0.0001 (C), F = 17.75 and p < 0.0001 (D), F = 15.58 and p < 0.0001 (E), F = 3.571 and p = 0.0417 (F), F = 8.750 and p = 0.0003 (G), F = 29.13 and p < 0.0001 (H). ∗∗∗, p < 0.001; ∗∗, p < 0.01, ∗, p < 0.05; and ns, not significant; Dunnett’s test.