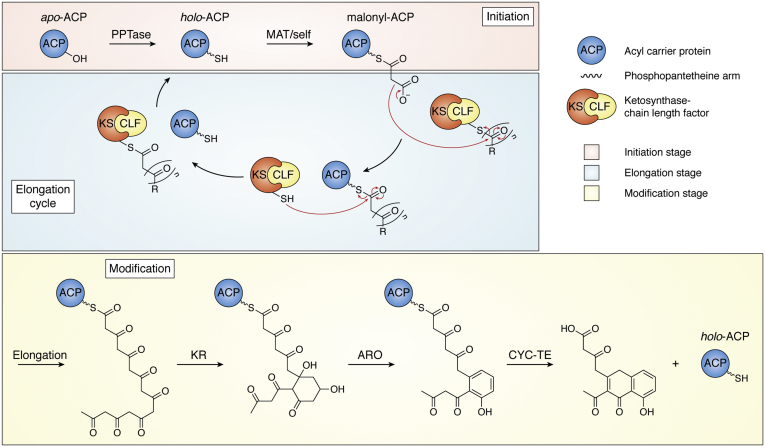
Figure 2.
ACPs play a central role in type II polyketide biosynthesis. The ACP is first activated by the PPTase-catalyzed attachment of the Ppant arm, which holds the polyketide intermediate (see Fig. 1A for structure of the Ppant arm). The Ppant arm is then acylated with malonyl-CoA by either interaction with MAT or self-malonylation. The subsequent elongation cycle adds two carbon units at a time to the growing β-keto chain. The growing chain is transferred to the KSCLF via a transacylation reaction, which makes the ACP available to participate in additional rounds of chain elongation. The general steps for the biosynthesis of the prototypical type II polyketide actinorhodin are outlined in this figure as an example. Type II PKSs tend to build C14-C30 β-keto chains (5), with the actinorhodin pathway involving a C16 chain. In actinorhodin biosynthesis, final modifications include the reduction of the C9 carbonyl catalyzed by the ketoreductase (KR) enzyme and ring closures catalyzed by aromatase (ARO) and cyclase/re (CYC), before release by the thioesterase (TE). ACP, acyl carrier protein; CoA, coenzyme A; KSCLF, ketosynthase chain length factor; PKS, polyketide synthase; Ppant, phosphopantetheine.