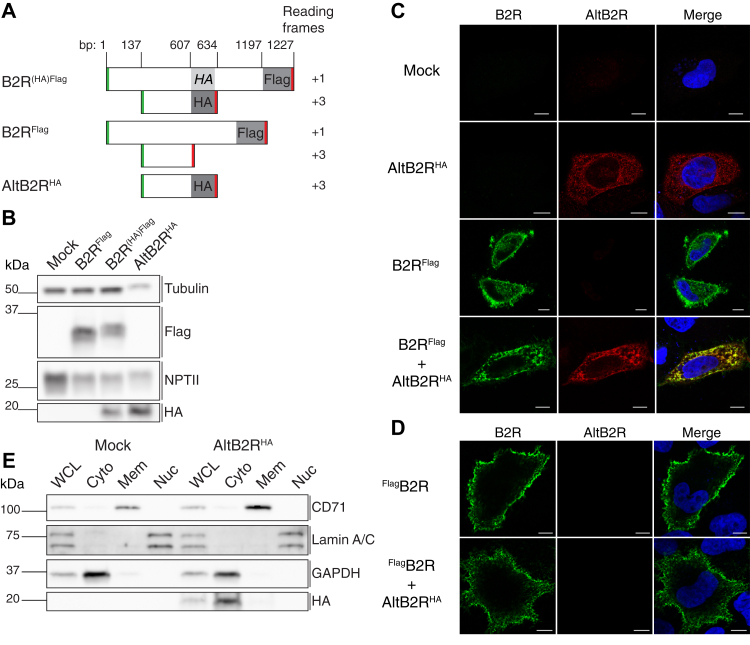
Figure 2.
AltB2R and B2R are coexpressed from the same coding sequence and colocalize in the cytoplasm and membranes of HeLa cells.A, strategy used to detect AltB2R and B2R. B2R with an in-frame C-terminal Flag tag, without and with an out-of-frame C-terminal HA tag on AltB2R within the coding sequence of B2R, and AltB2R with an in-frame C-terminal tag without the reference B2R. Positions of start and stop codons are indicated in green and red, respectively. Construction drawings are not to scale. B, detection of B2RFlag and AltB2RHA by western blotting using anti-Flag and anti-HA antibodies. Tubulin serves as a loading control and NPTII serves as a transfection control. Representative western blot of two independent experiments performed. C, subcellular localization of both B2RFlag and AltB2RHA by immunofluorescence using primary antibodies as in B. B2RFlag (green) and AltB2RHA (red) staining is shown in punctate granular vesicle-like structures within cytoplasm, perinuclear area, and underneath the plasma membrane. Scale bar: 10 μm. D, expression of B2R with an N-terminal Flag tag (FlagB2R) in live, nonpermeabilized cells confirmed localization at the plasma membrane. As expected, no immunofluorescent labeling of intracellular AltB2RHA was observed in nonpermeabilized conditions. Scale bar: 10 μm. C and D, representative single confocal images (midsection of cells) from three independent experiments. E, subcellular fractionation shows an enrichment of AltB2RHA in the cytoplasmic fraction compared with the whole cell lysat (WCL). Immunoblots of CD71 (plasma membrane; Mem), Lamin A/C (nucleus; Nuc), and GAPDH (cytoplasm; Cyto) were used to assess the purity of each fraction. Representative western blot of two independent experiments performed.