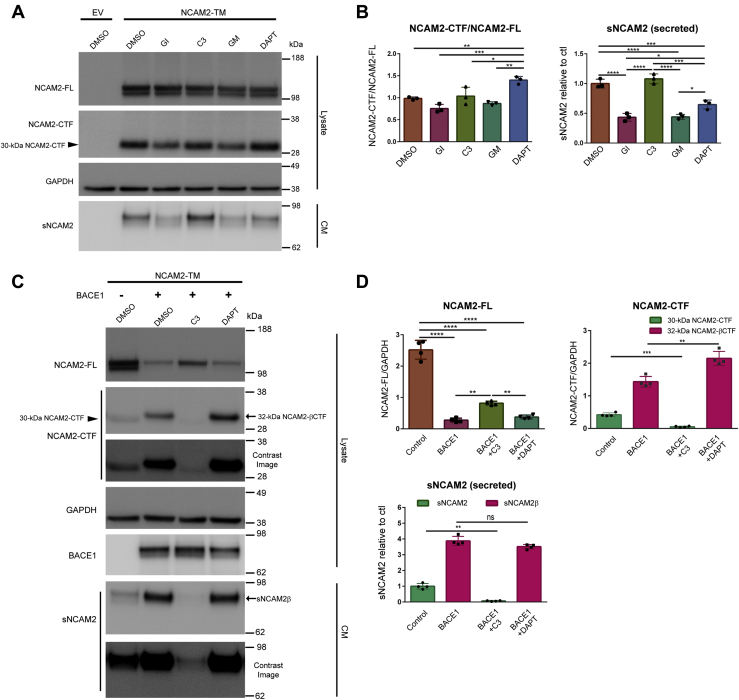
Figure 2.
NCAM2 is cleaved by metalloproteinases or BACE1, followed by γ-secretase in HEK cells. HEK cells were transfected with NCAM2-Myc-DDK expression vector (transmembrane NCAM2 isoform) or empty vector (EV) as a control and then treated with DMSO only, GI254023X (GI; selective ADAM10 inhibitor, 5 μM in DMSO), C3 (β-secretase inhibitor IV, 10 μM in DMSO), GM6001 (GM; broad spectrum of MMPs inhibitor, 2.5 μM in DMSO), and DAPT (γ-secretase inhibitor, 10 μM, in DMSO) for 24 h as indicated. Total DNA concentration was kept constant at 1.5 μg with empty vector. A, representative immunoblot of cell lysates (Lysate) and conditioned media (CM) using anti-Myc (2272), anti-NCAM2 (sc-136328), anti-BACE1 (D10E5), and anti-GAPDH (MAB374) antibodies. Ectopically expressed full-length NCAM2 (NCAM2-FL) in HEK cells undergoes proteolysis with the generation of a C-terminal fragment (NCAM2-CTF) in cell lysates and secreted soluble fragments (sNCAM2) in conditioned media. GI and GM treatments produced a significant reduction of sNCAM2 levels indicating that NCAM2 is cleaved by ADAM10 and MMPs. DAPT treatment results in the accumulation of NCAM2-CTF in cell lysates indicating that NCAM2-CTF is further processed by γ-secretase. C, HEK cells were cotransfected with NCAM2 and BACE1 or EV as control and then treated with solvent only (DMSO), C3, or DAPT for 24 h. The ectopic expression of BACE1 produces BACE1-specific NCAM2-CTF (NCAM2-βCTF) and soluble NCAM2 fragment (sNCAM2β), and their production is completely inhibited by C3 treatment. Note that a different protein ladder was used in this figure compared to Figure 1. B and D, graphs represent densitometric quantification of NCAM2-CTF/NCAM2-FL, NCAM2-FL/GAPDH, NCAM2-CTF/GAPDH ratio in lysates, and secreted soluble NCAM2 (sNCAM2 or sNCAM2β) in CM. One-way ANOVA with Tukey's multiple comparison test was applied. ∗p < 0.05, ∗∗<0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, A and B, n = 3; C and D, n = 4. Full-length versions of the western blots in C are shown in Figure S4A. ns, not significant.