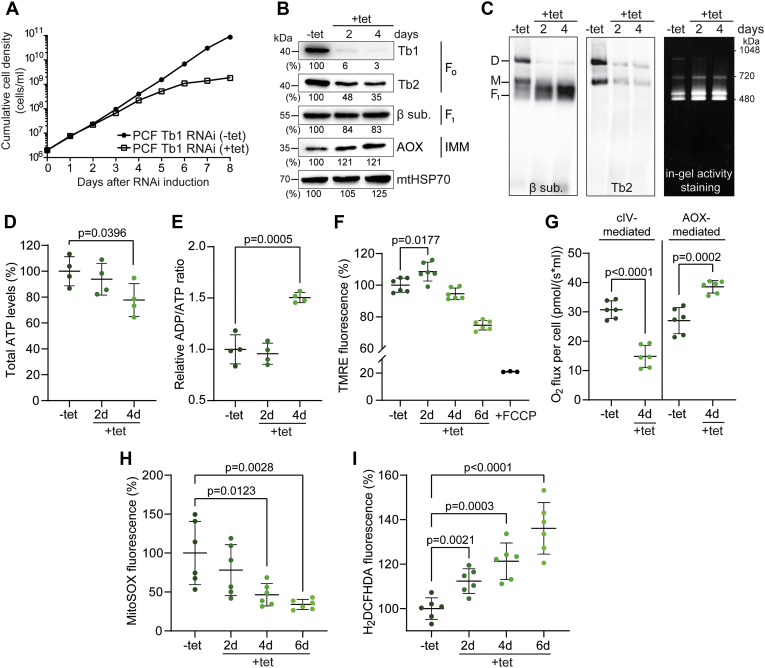
Figure 2.
Loss of Tb1 in PCF cells affects the structural integrity of the FoF1–ATP synthase complex and induces changes in mitochondrial physiology.A, growth of noninduced (−tet) and Tb1 RNAi–induced (+tet) PCF cells measured for 8 days. Cumulative cell density was calculated from the cell counts adjusted by the dilution factor needed to seed the cultures at 2 × 106 cells/ml each day. B, Western blot analysis of PCF Tb1 RNAi trypanosomes grown in the absence (−tet) or presence (+tet) of tetracycline for 2 and 4 days. Whole-cell lysates were subjected to SDS-PAGE followed by immunostaining with antibodies against Tb1 and Tb2 (Fo moiety), subunit β (F1 moiety), and AOX. The numbers beneath each blot represent the abundance of immunodetected protein expressed as a percentage of the noninduced sample after normalizing to the signal intensity of the mitochondrial HSP70 probing (loading control). C, BNE of 4 μg of DDM-lysed mitochondria from PCF Tb1 RNAi–noninduced (−tet) and PCF Tb1 RNAi–induced (+tet, 2 and 4 days) cells followed by Western blot analysis using antibodies against subunit β and Tb2 to detect free F1 and monomeric (M) and dimeric (D) ATP synthase complexes (first two panels). BNE of 60 μg of DDM-lysed mitochondria from PCF Tb1 RNAi–noninduced (−tet) and PCF Tb1 RNAi–induced (+tet, 2 and 4 days) cells followed by in-gel staining of ATPase activity (rightmost panel). D, total cellular ATP levels of PCF Tb1 RNAi–noninduced cells (−tet) and cells induced for 2 and 4 days (+tet, 2 days and 4 days) (means ± SD, n = 4, Student’s unpaired t-test). E, relative ADP/ATP ratio of PCF Tb1 RNAi–noninduced cells (−tet) and cells induced for 2 and 4 days (+tet, 2 days and 4 days) (means ± SD, n = 4, Student’s unpaired t-test). F, flow cytometry analysis of TMRE-stained PCF Tb1 RNAi–noninduced cells (−tet) and cells induced for 2, 4, and 6 days (+tet, 2 days, 4 days, and 6 days) to measure ΔΨm. The addition of FCCP served as a control for mitochondrial membrane depolarization (+FCCP) (means ± SD, n = 6, Student’s unpaired t-test). G, the oxygen consumption rate of PCF Tb1 RNAi live cells in the presence of glycerol-3-phosphate. After the addition of the substrate, cells were consuming oxygen at the steady rate. Injection of SHAM inhibited AOX-mediated respiration. The difference between the original values and the values after addition of SHAM is graphed as AOX-mediated respiration. Additional injection of KCN inhibited complex IV–mediated respiration and ceased the oxygen consumption of the cells. The difference between the values after SHAM addition and after KCN addition is graphed as complex IV–mediated respiration (means ± SD, n = 6, Student’s unpaired t-test). H and I, flow cytometry analysis of MitoSOX-treated (H) and H2DCFHDA-treated (I) PCF Tb1 RNAi–noninduced cells (−tet) and cells induced for 2, 4, and 6 days (+tet, 2 days, 4 days, and 6 days) to measure mitochondrial O2⋅− and total cellular ROS levels, respectively (means ± SD, n = 6, Student’s unpaired t-test). ΔΨm, mitochondrial membrane potential; AOX, alternative oxidase; BNE, blue native electrophoresis; DDM, dodecylmaltoside; FCCP, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone; H2DCFHDA, dichlorodihydrofluorescein; KCN, potassium cyanide; O2⋅−, superoxide; PCF, procyclic form; ROS, reactive oxygen species; SHAM, salicylhydroxamic acid; Tb1, ATPaseTb1; Tb2, ATPaseTb2; TMRE, tetramethylrhodamine ethyl ester.