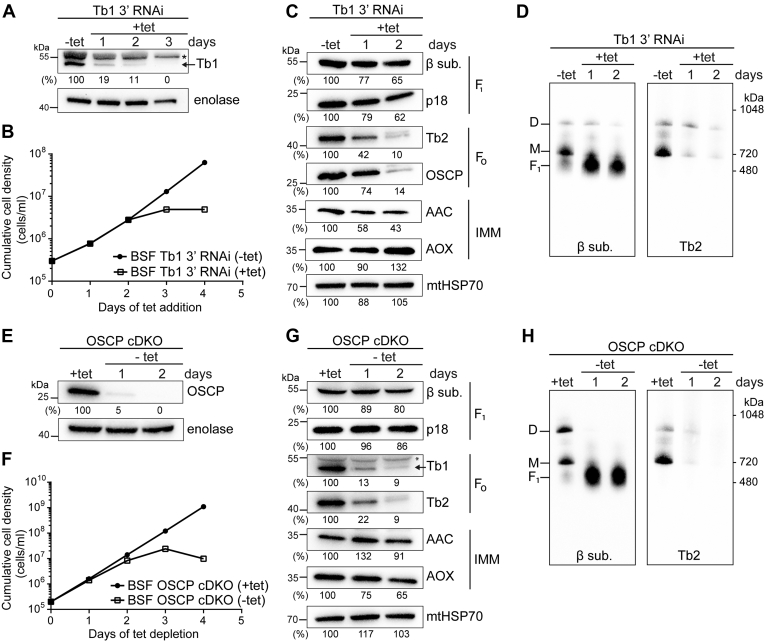
Figure 3.
Virtually complete loss of FoF1–ATPase is lethal to BSF cells.A, Western blot of whole-cell lysates from BSF Tb1 3’ RNAi–noninduced (−tet) and BSF Tb1 3’ RNAi–induced (+tet, 1–3 days) cells using an antibody against Tb1. The numbers beneath the blot represent the abundance of immunodetected Tb1 expressed as a percentage of the noninduced samples after normalizing to the signal intensity of the enolase probing (loading control). An asterisk points to a nonspecific band detected by anti-Tb1 antibody. B, growth of BSF Tb1 3’ RNAi–noninduced (−tet) and BSF Tb1 3’ RNAi–induced (+tet) cells measured for 4 days. Cumulative cell density was calculated from the cell counts adjusted by the dilution factor needed to seed the cultures at 2 × 105 cells/ml each day. C, Western blot analysis of whole-cell lysates from BSF Tb1 3’ RNAi–noninduced (−tet) and cells induced for 1 and 2 days (+tet) using antibodies against the F1 moiety (anti-β and anti-p18), the Fo moiety (anti-Tb2 and anti-OSCP), and inner mitochondrial membrane proteins (anti-AAC and anti-AOX). The immunoblot probed with anti-mitochondrial HSP70 antibody served as loading control. The densitometric analysis is depicted by the percentages beneath each blot and was carried out as in Figure 2B. D, BNE of 20 μg of DDM-lysed mitochondria from BSF Tb1 3’ RNAi–noninduced cells (−tet) and cells induced for 1 and 2 days (+tet) followed by Western blot analysis using antibodies to detect free F1 (anti-subunit β) and monomeric (M) and dimeric (D) FoF1–ATPase complexes (anti-Tb2). E, Western blot of whole-cell lysates from BSF OSCP cDKO cells grown in the presence (+tet) or absence (−tet) of tetracycline for 1 and 2 days using an antibody against OSCP. The numbers beneath the blot represent the abundance of immunodetected OSCP expressed as a percentage of the +tet sample after normalizing to the signal intensity of the enolase probing (loading control). F, the growth curve of BSF OSCP cDKO cells cultured in the presence (+tet) or absence (−tet) of tetracycline for 4 days. Cumulative cell density was calculated as in Figure 3B. G, Western blot analysis of whole-cell lysates from BSF OSCP cDKO cells cultured in the presence (+tet) or absence (−tet) of tetracycline for 1 day and 2 days using antibodies against the F1 moiety (anti-β and anti-p18), the Fo moiety (anti-Tb1 and anti-Tb2) and inner mitochondrial membrane proteins (anti-AAC and anti-AOX). The immunoblot probed with antimitochondrial HSP70 antibody served as the loading control. The densitometric analysis is depicted by the percentages beneath each blot and was carried out as in Figure 2B. The asterisk points to a nonspecific band detected by anti-Tb1 antibody. H, BNE of 20 μg of DDM-lysed mitochondria from BSF OSCP cDKO cells grown in the presence (+tet) or absence (−tet) of tetracycline for 1 day and 2 days followed by Western blot analysis using antibodies to detect free F1 (anti-subunit β) and monomeric (M) and dimeric (D) FoF1–ATPase complexes (anti-Tb2). AAC, ADP/ATP carrier; AOX, alternative oxidase; BNE, blue native electrophoresis; BSF, bloodstream form; cDKO, conditional double knock-out; DDM, dodecylmaltoside; OSCP, oligomycin sensitivity-conferring protein; Tb1, ATPaseTb1; Tb2, ATPaseTb2.