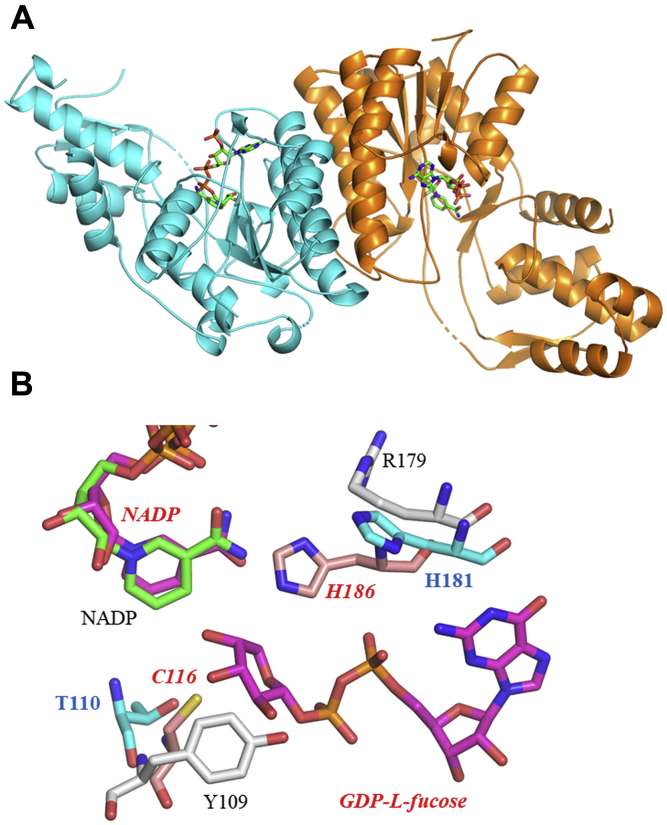
Figure 10.
Structural characterization of the reductases DdahC and MlghC.A, the dimer of DdahC shown as a cartoon, with one monomer in orange and the other in cyan. NADP is shown as sticks with carbons green, oxygen red, nitrogen blue, and phosphorous orange. B, the active site of MlghC and DdahC, with NADP colored as in panel A. Amino acid side chains are labeled and shown with carbon atoms of MlghC colored gray (Y/R dyad) and of DdahC in blue (T/H dyad). The C/H catalytic dyad of GFS (RCSB 1BWS) is shown with side chains in salmon and labeled in red. GDP-L-fucose from the GFS structure is also shown (labeled in red). GFS, GDP-L-fucose synthase.