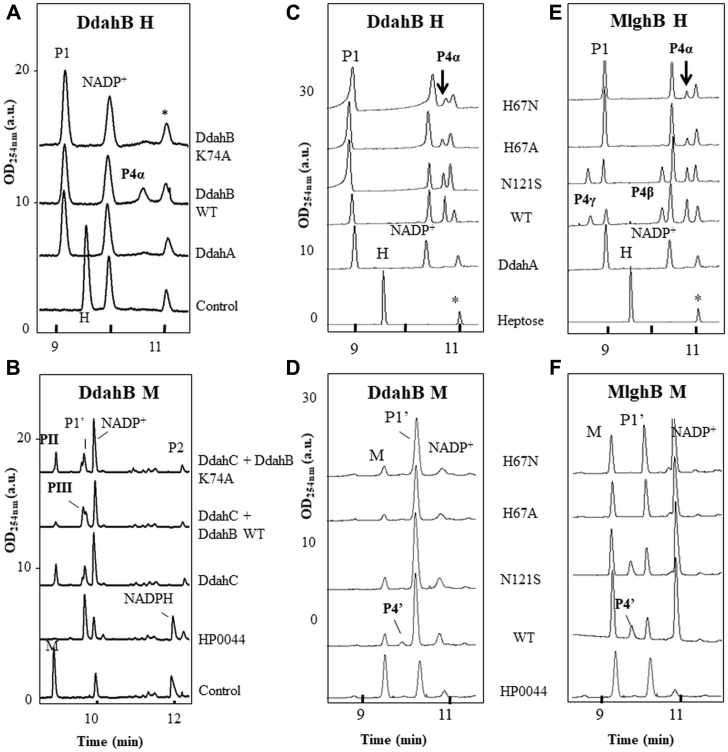
Figure 4.
Catalytic activity of K74A, H67N/A, and N121S DdahB and/or MlghB.A, reactions containing 23.2-μM DdahA, 0.2-mM GDP-manno-heptose (H), 0.2-mM NADP+, and 0.4-μM DdahB were incubated for 45 min. ∗ denotes an impurity present in the heptose preparation and that serves as an internal standard. The reaction product P4α is observed for WT DdahB but not for K74A mutated DdahB. B, reactions containing 5-μM HP0044, 1-mM GDP-mannose (M), and 1-mM NADPH/+ (50/50 %/%) were incubated for 2 h to convert all mannose in P1’ before incubation with 4-μM DdahB and 1 μM of DdahC for 5 h. DdahC was added to stabilize any P4’ generated by DdahB. Peaks PII and PIII correspond to DdahC reduction products, stemming from P1’ and P4’, respectively, as demonstrated later in the study. Formation of PIII is observed in reactions comprising WT DdahB, but not K74A DdahB, indicating impaired P4’ formation by K74A DdahB. C and E, reactions containing 0.17-mM heptose, 1.5-μM DdahA, 0.13-mM NADP+, and 0.1-μM epimerase were incubated for 30 min. The effect of the mutations is assessed by comparing the amount of epimerization product formation (P4α, P4β, P4γ) between mutated and WT enzymes. D and F, a 50-μl reaction containing 0.77-mM mannose and 0.2-μM HP0044 was incubated for 90 min to generate P1’ (∼50% conversion obtained). Aliquots of 8 μl were supplemented with 0.5-μM (final concentration) of DdahB and 0.1-mM NADP+, 0.1 μM of MlghB and 0.5-mM NADP+, or none and incubated for 5 h. The higher concentration of DdahB used accounts for its low catalytic efficacy on mannose. The effect of the mutations is assessed by comparing the amount of epimerization product formation (P4’) between mutated and WT enzymes. All reactions were set up in duplicates. Figure 4 shows a representative example of each reaction. Quantitation and kinetics are shown in Fig. S2.