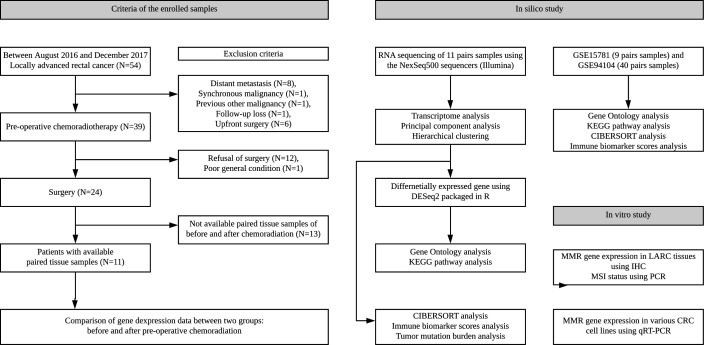
Figure 1.
Diagram of patient selection and study workflow. Samples before neoadjuvant CRT were obtained at the time of diagnosis using endoscopy. Samples after neoadjuvant CRT were obtained after surgical resection. Samples were obtained from 11 patients after enrollment and were prepared and used for RNA sequencing analysis. The 11 patients underwent curative surgery. CRC, colorectal cancer; CRT, chemoradiation therapy; IHC, immunohistochemistry; KEGG, Kyoto Encyclopedia of Genes and Genomes; LARC, locally advanced rectal cancer; MMR, mismatch repair; MSI, microsatellite instability.