Abstract
Microbial wound contamination is known to be a hindrance to wound healing. Negative pressure wound therapy (NPWT) with or without irrigation is known to optimise conditions in problem wounds. The aim of this study was to investigate the influence of computer‐controlled wound irrigation with NPWT on the bacterial load in contaminated wounds. A total of 267 patients were treated with NPWT with automated instillation because of problematic wounds using an antiseptic instillation solution. In 111 patients, a minimum of 4 operative procedures were necessary, and swabs were taken at least at the first and at the fourth operation in a standardised procedure. The number of different bacteria and the amount of bacteria were analysed during the course. In a subgroup of 51 patients, swabs were taken at all 4 operative procedures and analysed separately. In an overall analysis, the number of different bacteria and the amount of bacteria significantly decreased independent of wound localisation and diagnosis. NPWT with automated instillation demonstrates a positive influence in the reduction of bacterial load in problem wounds. Thus, it may help to optimise wound conditions before definite wound closure.
Keywords: bacterial load, contaminated wounds, instillation, negative pressure wound therapy
1. INTRODUCTION
There is no doubt that microbial contamination of wounds is a hindrance to wound healing. It is known as a frequent source of failure or secondary problems when closing defect wounds with reconstructive surgery because of a higher rate of surgical site infections. Microbial wound colonisation of infected tissue can be characterised by the presence of multiplying microorganisms in the depth or on the surface of a wound. Microbial colonisation is characterised by the presence of microorganisms on a wound, showing no immune response from the host and no associated clinical signs or symptoms. In contrast, wound infection depends on the pathogenicity and virulence of the microorganisms and on the immune competency of the host. It is determined by the presence of clinical signs of infection, such as erythema, pain, tenderness, heat, oedema, cellulites, and abscess/pus.1 Topical negative pressure wound therapy (NPWT) is 1 means to achieve appropriate wound bed conditioning and a reduction of bacterial load.2, 3, 4 It has also been shown to positively influence time to healing in high risk wounds when compared with conventional remedies in an early phase of treatment.5 An enormous amount of clinical data on various cohort studies, case series, and meta‐analyses have been reported in the literature claiming various positive clinical effects of topical negative pressure therapy on open and complicated wounds.5, 6, 7, 8, 9 It has been reported to reduce the number of dressing changes, patient‐related pain, and the length of hospital stay and also to alter molecular mechanisms at the genetic level.10, 11, 12 Most recently, the effects of altering the skin perfusion and reducing strain on wound edges has led to the concept of NPWT over closed incisions.13, 14
Increasing insight into the wound biofilm problem has also led to the demand among surgeons to optimise traditional irrigational treatment of infected and contaminated wounds.15 Computerised automated devices have been developed in order to clean infected wounds more thoroughly before definitive coverage is performed. Interestingly, it was demonstrated in experimental wounds that instillation in combination with NPWT accelerates the induction of granulation tissue up to 43% when compared with NPWT alone.16 Although the type of instillation solution is a matter of ongoing debate, several studies have shown that topical NPWT with automated instillation (NPWTi) helps to treat infected wounds.17, 18, 19, 20
As there is a lack of data, we investigated patients with wounds of different origins and at various body sites to determine whether or not repeated cycles of instillation with a standardised treatment protocol may reduce the number and/or the bacterial load in problem wounds.
2. METHODS
Patients treated with NPWTi between January 2013 and November 2017, with wounds of different origins at various body sites, were analysed in an open prospective study. The protocol was approved by the institutional Review Board (registration number 4207) in accordance with the Declaration of Helsinki. Inclusion criteria for this study were different types of ulcers; chronic, acute and trauma‐related wounds with an indication for NPWTi; and written informed consent provided by the patients. Another prerequisite for the study and the use of NPWTi was contamination of the wound, as was expected in chronic wounds or severe soft tissue defects. To investigate the influence of the instillation therapy over a longer period, patients who had undergone a minimum of 4 operative interventions were included in this study and therefore offered at least 1 swab at the first and 1 swab at the last surgical intervention to analyse any change in the bacterial load. To obtain information more precisely on potential changes in the bacterial load, swabs were collected in a subgroup during all 4 operations. After initial surgical debridement and application of the NPWTi dressing, changes of the dressing and further wound debridement, if necessary, were performed before the definitive reconstructive procedure.
In a standardised fashion, swabs were removed from the tube and firmly rubbed and rolled in a circular movement several times across the sampling area before the disinfection and the debridement. This was continued until the whole surface had been wiped. Swab samples were collected for culture analysis and immediately sent to the laboratory. They were analysed for the number of the oftentimes numerous and different bacterial species, including all types of germs found in the wound (number of different bacterial species = NDB). To further clarify a potential effect of instillation therapy, we also recorded the semi‐quantitative measure of the gross cultural microbiological description of the amount of counted bacteria under culture according to the routine procedure of the Institute of Microbiology and Hygiene University Hospital Erlangen. This measure allows a rough estimation (non‐absolute values) and is frequently used in clinical routine to judge the severity of bacterial colonisation with a pseudo‐numerical categorisation of sparse,1 moderate,2 several,3 or plenty of germs4 (Amount of Bacteria [AB]). Because of the heterogeneity of the wounds and their bacterial colonisation, the average of the amount of all bacteria found in the wound was calculated. The number of different bacteria (NDB) and AB of the first and last swab from all patients and of all 4 operations in the subgroup were recorded separately to determine any changes during the therapy. The perioperative single‐shot antibiotic prophylaxis was administered after the aforementioned sampling procedure of swabbing had been performed. Furthermore, the last cycle of irrigation was at least 2 hours or longer before the surgical intervention and the swab sampling to minimise the rate of false‐negative results. Final surgical reconstruction was performed when the wounds were clinically deemed clean enough for coverage, showing no more signs of newly developed necrotic or infected tissue, ideally presenting homogenous granulation tissue. This was possible in all cases, either with flaps, skin grafts, secondary wound closure, or wound healing by secondary intention. The decision to close the wound was based on the clinical aspect by the surgeon and not on the bacterial analysis.
All patients received an intravenous treatment with appropriate antibiotics after flap or graft reconstruction. In cases of abscesses, the patients received ongoing antibiotics as the only subgroup. Additional data, such as gender, age at time of operation, hospital stay, and anatomical localisation of the wound, were also analysed.
NPWTi was applied to all wounds in a similar manner for each patient. Depending on the location and wound severity, dressing was changed under local or general anaesthesia up to 3 times a week. Heavily infected wounds required dressing changes more frequently. Following careful disinfection, surgical wound debridement was performed, including sufficient excision of all necrotic and non‐viable tissue and bone. After adequate haemostasis had been achieved, the surrounding skin was cleaned, and the NPWTi Dressing Kit was placed on the wound and connected to the device. To prevent direct contact between the polyurethane foam and vessels, nerves, or organs, a permeable protecting gauze was inserted if necessary. The use of circumferential dressings was avoided. The vacuum was set on −125 mm Hg. As an antiseptic solution, 0.4 mg/1 mL of polihexanide (Lavasept®, B. Braun Medical AG, Germany) was used for instillation. The instillation volume was set according to the wound size and depth. Delivery and removal of the antiseptic to the wound was performed by the fully automated device with a dwell time of 20 minutes for each patient and repeated cycles every 2 hours.
The Wilcoxon signed‐rank test was used for statistical evaluation comparing with related samples. The significance level was set at P = .05.
3. RESULTS
In the period from January 2013 to November 2017, we investigated a total of 267 patients who were treated with NPWTi. Of these, 148 patients (55.4%) had 4 or more surgical interventions, and 111 patients (41.6%) met the inclusion criteria for this study; 45 female (40.5%) and 66 male (59.5%) patients were included. The average age was 58.6 years (range 20‐86 years). Average duration of NPWTi was 11.5 days (SD 3.9 days), with a range of 7 up to 31 days. Mean hospital stay was 22.6 days (SD 8.6 days), ranging from 10 to 54 days.
Wounds were localised in 17.1% at upper extremities, 52.3% at lower extremities, and 30.6% at the trunk. Wounds treated with NPWTi were postoperative infections 10.8% (n = 12 patients), osteomyelitis 11.7% (n = 13 patients), chronic ulcers 10.8% (n = 12 patients), chronic wounds at different locations 12.6% (n = 14 patients), necrosis 4.5% (n = 5 patients), wounds after trauma 8.1% (n = 9 patients), abscesses 20.7% (n = 23 patients, defined as invasive infections), and pressure ulcers 9.0% (n = 10 patients), followed by wounds after tumour excision 1.8% (n = 2 patients) (Figure 1). Further indications (9.9%, n = 11 patients) for NPWTi were any wounds with higher potential of infectivity, such as local excisions of hidradenitis suppurativa.
Figure 1.
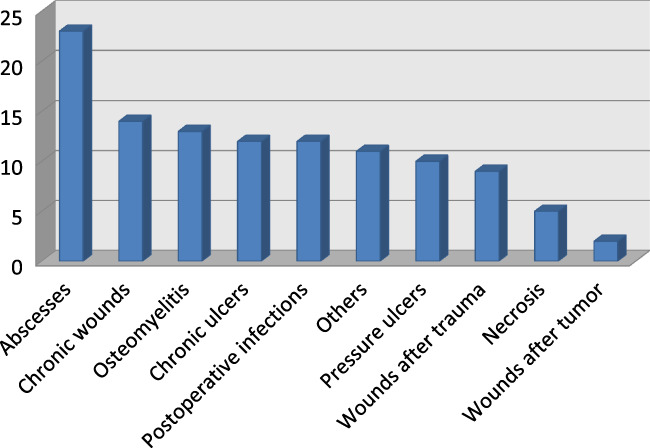
Distribution of patients according to dependence of wound entity (y‐axis number of patients) [Colour figure can be viewed at wileyonlinelibrary.com]
Final wound reconstruction, including local or free flaps 49.5% (n = 55 patients), skin grafts 28.8% (n = 32 patients), or secondary wound closure 18.0% (n = 20 patients), was performed in 96.4% of all cases. In the other cases, secondary healing was intended in 3.6% (n = 4 patients).
The average of the NDB of all 111 patients was 1.7 NDB at the first operation, with a range of 0 up to 6 different bacteria. In 41.5% (n = 46), 2 or more different bacteria were found, and in 43.2% (n = 48) and in 15.3% (n = 17), 1 and no bacteria were found at all, respectively. At the fourth operation, a decrease of the mean number of 48% to 0.9 was observed (P < .001). In 22.5% (n = 25), 2 or more different bacteria were found, 1 in 28.8% (n = 32), and no verifiable bacteria in 48.7% (n = 54) at this time. The AB of each swab was documented as described above. The mean AB was 4.2 AB at the first operation, with a maximum of 13. At the fourth operation, the mean AB showed a significant (P < .001) decrease of 65% to 1.5 AB.
The distribution of microbial colonisation depending on diagnosis showed a maximum of bacterial load for pressure ulcers, chronic wounds at different locations, and chronic ulcers. During NPWTi, a reduction of the mean bacterial number to 46% for pressure ulcers, 22% for chronic wounds at different locations, and 56% for chronic ulcers was observed. Figure 2 shows the reduction of bacterial number and Figure 3 the reduction of the mean AB depending on the different diagnosis.
Figure 2.
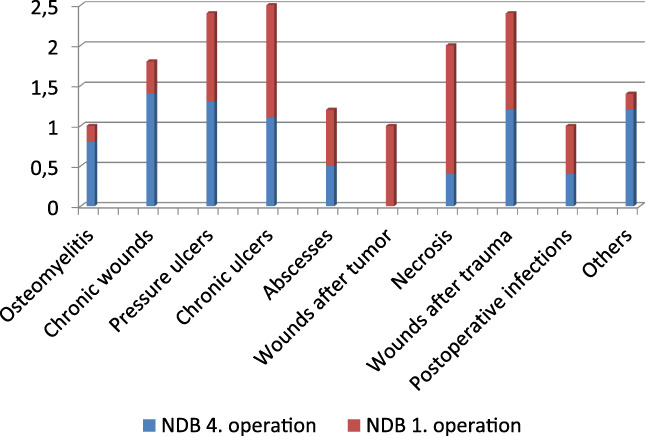
Reduction of the number of different bacteria (NDB) in different wounds under negative pressure wound therapy with automated instillation (NPWTi) during the course from the first to the fourth operation (y‐axis number of different bacteria) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
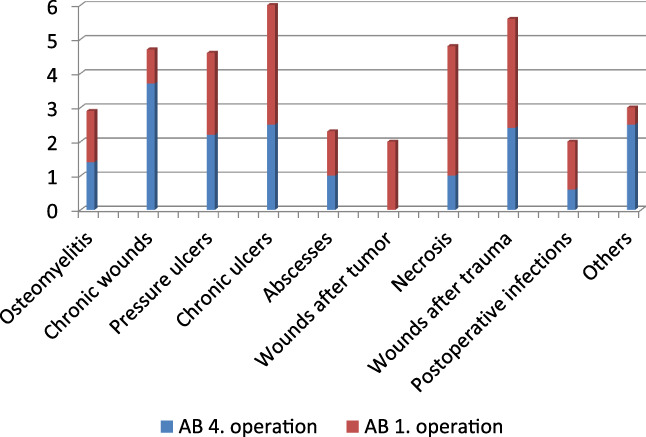
Reduction of the amount of bacteria (AB) in different wounds under negative pressure wound therapy with automated instillation (NPWTi) during the course from the first to the fourth operation (y‐axis AB) [Colour figure can be viewed at wileyonlinelibrary.com]
Of 111 patients, 51 were eligible for a repeated bacterial load follow up over at least 4 cycles of NPWTi. In this subgroup, we treated 15.7% (n = 8 patients) with postoperative infections; 21.6% (n = 11 patients) with osteomyelitis; 13.7% (n = 7 patients) with chronic ulcers; 7.8% (n = 4 patients) with necrosis, wounds after trauma, and abscesses; 9.8% (n = 5 patients) with pressure ulcers; and 3.9% (n = 2 patients) with wounds after tumour excision (Figures 4 and 5). A total of 11.7% patients (n = 6) were treated for other indications as described.
Figure 4.
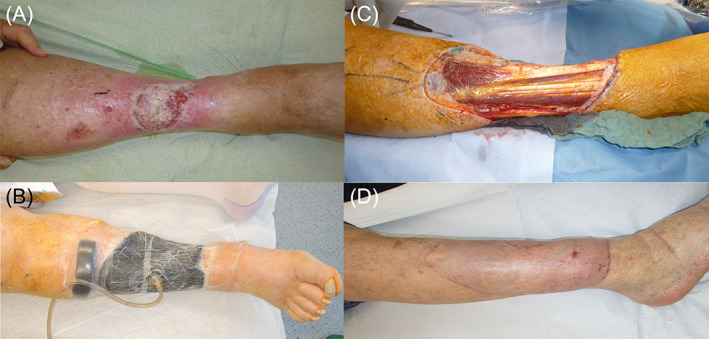
A, Combination of a basal cell carcinoma and a squamous cell carcinoma at the lower right leg. B, Lower right leg with negative pressure wound therapy with automated instillation (NPWTi). C, Wound at the lower right leg after tumour resection. D, Lower right leg after wound reconstruction with a free microvascular latissimus dorsi flap [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 5.
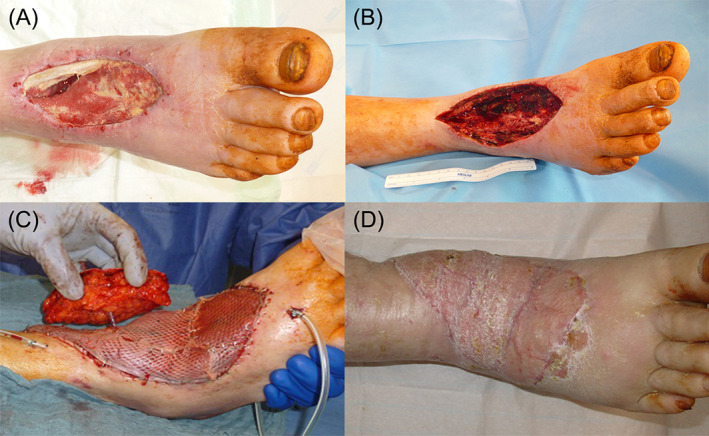
A, Chronic wound right foot after abscess incision 4 month ago at the time of admission to our department. B, Wound on the right foot after initial debridement. C, Defect reconstruction with a free rectus abdominis flap with perforator based skin island for postoperative flap monitoring. D, Right foot 6 months after defect reconstruction [Colour figure can be viewed at wileyonlinelibrary.com]
The results show a continuous reduction of the mean bacterial number as well as of the mean AB in nearly all groups. In the groups with initially purulent infections and in wounds after tumour excision, no more bacteria could be found in the swab at the fourth operation. For patients with wounds because of osteomyelitis, the reduction of NDB was 40% and 61% for AB. An effective reduction for NDB and AB was also found in chronic ulcers showing a decrease of 61% and 69%, respectively. In patients suffering from pressure ulcers, NDB and AB presented minimally higher at the fourth operation, with an increase of 16% and 3%, respectively. All pressure ulcers were located sacral in proximity to the patient's anus. Only one patient in this small group (n = 5) demonstrated an increase of NDB and AB. This patient was admitted to the hospital in poor condition and developed a sepsis during the course. Of the group, 2 patients showed an unchanged NDB and 2 patients a decrease of NDB, whereas AB dropped in 3 of the patients and presented unchanged in 1 patient.
During all operations, the analysis of average NDB and AB of all 51 patients demonstrated a decrease of both parameters at every single operation. Bacterial number showed 1.7 NDB at the first operation, 1.2 NDB at the second, 1.0 NDB at the third, and 0.9 at the fourth. A similar course was observed for the AB, with 3.8 AB at the first operation, 2.4 AB at the second, 2.0 AB at the third, and 1.7 AB at the fourth (Figure 6). A shift of bacteria from Gram‐positive to Gram‐negative or vice versa was not observed.
Figure 6.
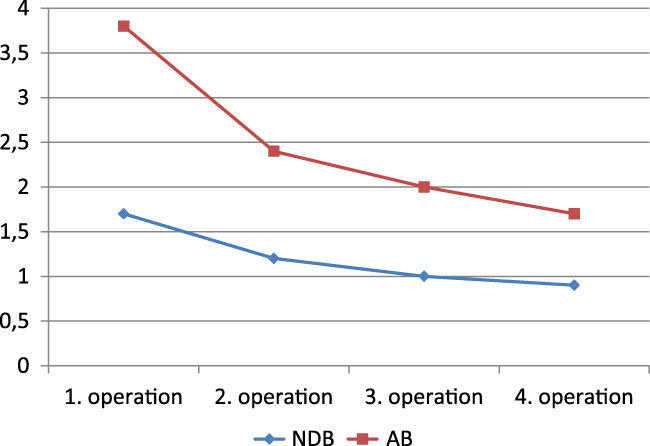
Development of the number of different bacteria (NDB) and the amount of bacteria (AB) under negative pressure wound therapy with automated instillation (NPWTi) during the course of 4 operations (analysis of swabs at each operation) [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Several studies focused on the details and potential beneficial effects of instillation therapy combined with NPWT to treat hard‐to‐heal wounds.21, 22, 23 It is still a matter of ongoing debate whether antiseptic solutions or simple saline should be preferred for wound irrigation as well as for the optimal dwell time and number of cycles per day and the necessary overall treatment duration.21, 24, 25, 26 The influence of what has been termed “micro‐debridement” by repetitive wound washing may be independent from the type of irrigating fluid.27, 28 Especially when biofilm infected hardware cannot be easily removed without severe damage to the patient, the use of antimicrobial substances has been shown to be helpful in salvaging devices or vital tissue.29, 30 In our study, we used an antiseptic, which is the standard solution for wound irrigation in our daily routine and which would potentially allow a comparison with our previous treatment results without NPWT. In our study, we excluded wounds with exposed devices or hardware because this appears to be another entity because of the biofilm problem.
It is common sense that, optimally, in an exact volumetric assessment of the bacterial load, using defined biopsies would be the most exact tool to measure colonisation rates. While this is true, it does not necessarily reflect the daily clinical practice in the real world and was not our primary goal. Because of the various localisations and origins of the wounds, which the standard patient population of a plastic surgical university unit comprises, punch biopsies would not have been possible in most patients with delicate wounds often exposing vital structures, such as nerves or vessels. The intention of our study was to simulate the common clinical practice with standardised swabbing in daily clinical routine. Although one might argue that, during the clinical course of repeated dressing changes, an effect of the antimicrobial solution on the swab sampling could have been part of the microbial reduction, this argument would not counteract the validity of our findings. This is because the conditions were equal for all wounds and at all times. Semi‐quantitative bacterial counts are common clinical practice and, for the purpose of this study, therefore reflect what is probably close to the most frequently performed daily routine. The reduction of the number as well as of the AB during NPWTi was independent from the patients' gender, wound localisation, and aetiology. These results appear to indicate a positive influence of NPWTi on the wound bed colonisation, which should facilitate an earlier and more successful plastic reconstruction according to current conceptions and could contribute to a reduction in surgical site infections. This might influence the duration of a necessary hospital stay. In our study, we did not intend to compare different irrigational solutions in a control group. Several studies have shown favourable effects using saline without any antiseptics.27 There is also no doubt that a repeated surgical debridement, which was performed in all our cases, has a crucial influence on the bacterial burden of any wound.31, 32 However, until now, it has not been possible to determine the exact influence of either the surgical therapy or the NPWTi on the overall bacterial reduction. We suppose that repeated radical wound debridements play a significant role, but our data also undermine a potential effect of NPWTi with antiseptics.
In addition, all patients in this survey received intravenous treatment with appropriate antibiotics during the therapy, as a perioperative prophylaxis, and finally when the wound was covered. However, because it is well known that microbial biofilms are not responsive to systemic antibiotic treatment and at least do reduce the antibiotic effects, it can be assumed that the antibiotic impact on superficial infected wounds with biofilm can be neglected. This is especially true when, because of poor microcirculation, an adequate tissue perfusion of the affected tissue is not given, and systemic antibiotics cannot penetrate into poorly vascularised infected wounds. Nevertheless, NPWTi could have a positive effect with repetitive antiseptic irrigation as it has been demonstrated to prevent bacterial increase. In some patients, we clinically observed a more pronounced granulation of the wound bed compared with what we had previously seen with similar wounds with NPWT without instillation.
Although NPWTi and its system, especially the foam, are designed to treat deep and difficult‐to‐reach areas, it remains unclear if extremely deep and fistulating wounds, such as deep pressure ulcers or osteomyelitic bone defects, can be completely rinsed without potentially omitting some spots in the depth. Especially in wounds such as sacral pressure ulcers, the proximity to the anus and therefore the physiologically increased bacterial load compared with other anatomic regions are often problematic. Furthermore, those anatomic regions are sometimes challenging to the application of NPTWi or NPTW as vacuum or solution leakage can occur. It may be difficult to establish adequate wound models to test the dilution of antiseptics in 3‐dimensional deep and fissured sore caves.33 The influence of the physical structure of open cell foams as commonly used for NPWT appears to allow a thorough penetration of the irrigational fluid to the wound. Nevertheless, altering the physical properties and pore sizes could eventually optimise the cleansing capacity of the systems used for NPWTi. As surgical revision allows for additional lavage during foam changes, even deep wounds should not be excluded from instillation therapy when the indications appear appropriate.
5. CONCLUSION
Our study demonstrates a reduction in the number as well in the amount of bacterial colonisation/bioburden of wounds under the influence of NPWTi. This may improve clinical outcomes in the treatment of problem wounds. To increase the level of the present evidence, randomised controlled trials should be implemented to further clarify the optimal conditions, such as dwell time, type of irrigant, etc. While surgical debridement will always play a key role, NPWTi may help to optimise wound conditions before definitive closure.
ACKNOWLEDGEMENTS
Parts of this study were performed to fulfil the requirements of obtaining the doctoral title “Dr. med.”.
Ludolph I, Fried FW, Kneppe K, Arkudas A, Schmitz M, Horch RE. Negative pressure wound treatment with computer‐controlled irrigation/instillation decreases bacterial load in contaminated wounds and facilitates wound closure. Int Wound J. 2018;15:978–984. 10.1111/iwj.12958
REFERENCES
- 1. Bessa LJ, Fazii P, Di Giulio M, Cellini L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: some remarks about wound infection. Int Wound J. 2015;12(1):47‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47(5):547‐551. [DOI] [PubMed] [Google Scholar]
- 3. Horch RE, Nord D, Augustin M, Germann G, Leffler M, Dragu A. Economic aspects of surgical wound therapies. Chirurg. 2008;79(6):518‐525. [DOI] [PubMed] [Google Scholar]
- 4. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553‐562. [DOI] [PubMed] [Google Scholar]
- 5. Yao M, Fabbi M, Hayashi H, et al. A retrospective cohort study evaluating efficacy in high‐risk patients with chronic lower extremity ulcers treated with negative pressure wound therapy. Int Wound J. 2014;11(5):483‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falagas ME, Tansarli GS, Kapaskelis A, Vardakas KZ. Impact of vacuum‐assisted closure (VAC) therapy on clinical outcomes of patients with sternal wound infections: a meta‐analysis of non‐randomized studies. PLoS One. 2013;8(5):e64741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holle G, Germann G, Sauerbier M, Riedel K, von Gregory H, Pelzer M. Vacuum‐assisted closure therapy and wound coverage in soft tissue injury. Clinical use. Der Unfallchirurg. 2007;110(4):289‐300. [DOI] [PubMed] [Google Scholar]
- 8. Schintler MV. Negative pressure therapy: theory and practice. Diabetes Metab Res Rev. 2012;28(suppl 1):72‐77. [DOI] [PubMed] [Google Scholar]
- 9. Willy C, Gerngross H. Scientific background of the vacuum closure—an abstract. Zentralbl Chir. 2004;129(suppl 1):S6. [DOI] [PubMed] [Google Scholar]
- 10. Koncar I, Cvetkovic S, Dragas M, et al. Vacuum‐assisted wound closure in vascular surgery—clinical and cost benefits in a developing country. Vojnosanit Pregl. 2016;73(1):9‐15. [DOI] [PubMed] [Google Scholar]
- 11. Leffler M, Derrick KL, McNulty A, Malsiner C, Dragu A, Horch RE. Changes of anabolic processes at the cellular and molecular level in chronic wounds under topical negative pressure can be revealed by transcriptome analysis. J Cell Mol Med. 2011;15(7):1564‐1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Jesus LE, Martins AB, Oliveira PB, Gomes F, Leve T, Dekermacher S. Negative pressure wound therapy in pediatric surgery: how and when to use. J Pediatr Surg. 2018;53(4):585‐591. [DOI] [PubMed] [Google Scholar]
- 13. Dragu A, Schnurer S, Unglaub F, et al. Wide topical negative pressure wound dressing treatment for patients undergoing abdominal dermolipectomy following massive weight loss. Obes Surg. 2011;21(11):1781‐1786. [DOI] [PubMed] [Google Scholar]
- 14. Horch RE. Incisional negative pressure wound therapy for high‐risk wounds. J Wound Care. 2015;24(4 suppl):21‐28. [DOI] [PubMed] [Google Scholar]
- 15. Lehner B, Fleischmann W, Becker R, Jukema GN. First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study. Int Orthop. 2011;35(9):1415‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lessing MC, James RB, Ingram SC. Comparison of the effects of different negative pressure wound therapy modes‐continuous, noncontinuous, and with instillation‐on porcine excisional wounds. ePlasty. 2013;13:e51. [PMC free article] [PubMed] [Google Scholar]
- 17. Gabriel A, Kahn K, Karmy‐Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost‐effectiveness. ePlasty. 2014;14:e41. [PMC free article] [PubMed] [Google Scholar]
- 18. Gabriel A, Shores J, Heinrich C, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008;5(3):399‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brinkert D, Ali M, Naud M, Maire N, Trial C, Teot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J. 2013;10(Suppl 1):56‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fluieraru S, Bekara F, Naud M, et al. Sterile‐water negative pressure instillation therapy for complex wounds and NPWT failures. J Wound Care. 2013;22(6):293‐294. 296, 298, 299. [DOI] [PubMed] [Google Scholar]
- 21. Willy C. Breakthrough ideas leading to new futures: prologue. Int Wound J. 2013;10(suppl 1):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludolph I, Lehnhardt M, Arkudas A, et al. Plastic reconstructive microsurgery in the elderly patient ‐ Consensus statement of the German Speaking Working Group for Microsurgery of the Peripheral Nerves and Vessels. Handchir Mikrochir Plast Chir. 2018;50(2):118‐12. [DOI] [PubMed] [Google Scholar]
- 23. Gunter G. Complication management is risk management is reduction of loss ratio: a personal stance. Handchir Mikrochir Plast Chir. 2017;49(4):229‐233. [DOI] [PubMed] [Google Scholar]
- 24. Brem MH, Blanke M, Olk A, et al. The vacuum‐assisted closure (V.A.C.) and instillation dressing: limb salvage after 3 degrees open fracture with massive bone and soft tissue defect and superinfection. Unfallchirurg. 2008;111(2):122‐125. [DOI] [PubMed] [Google Scholar]
- 25. Back DA, Scheuermann‐Poley C, Willy C. Recommendations on negative pressure wound therapy with instillation and antimicrobial solutions—when, where and how to use: what does the evidence show? Int Wound J. 2013;10(suppl 1):32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raad W, Lantis JC II, Tyrie L, Gendics C, Todd G. Vacuum‐assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int Wound J. 2010;7(2):81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teot L, Boissiere F, Fluieraru S. Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int Wound J. 2017;14(5):842‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh DP, Gowda AU, Chopra K, et al. The effect of negative pressure wound therapy with antiseptic instillation on biofilm formation in a porcine model of infected spinal instrumentation. Wounds. 2017;28(6):175‐180. [PubMed] [Google Scholar]
- 29. Meybodi F, Sedaghat N, French J, Keighley C, Mitchell D, Elder E. Implant salvage in breast reconstruction with severe peri‐prosthetic infection. ANZ J Surg. 2017;87(12):E293‐E299. [DOI] [PubMed] [Google Scholar]
- 30. Deleyto E, Garcia‐Ruano A, Gonzalez‐Lopez JR. Negative pressure wound therapy with instillation, a cost‐effective treatment for abdominal mesh exposure. Hernia. 2018;22(2):311‐318. [DOI] [PubMed] [Google Scholar]
- 31. Kirsner RS, Romanelli M. Use of advanced technologies across the wound care spectrum: prologue. Int Wound J. 2016;13(suppl 3):5‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta S, Gabriel A, Lantis J, Teot L. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int Wound J. 2016;13(2):159‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matiasek J, Domig KJ, Djedovic G, Babeluk R, Assadian O. The effect of negative pressure wound therapy with antibacterial dressings or antiseptics on an in vitro wound model. J Wound Care. 2017;26(5):236‐242. [DOI] [PubMed] [Google Scholar]


